Figure 8. Model for caspase-9 conformational states in the presence of CARD domain.
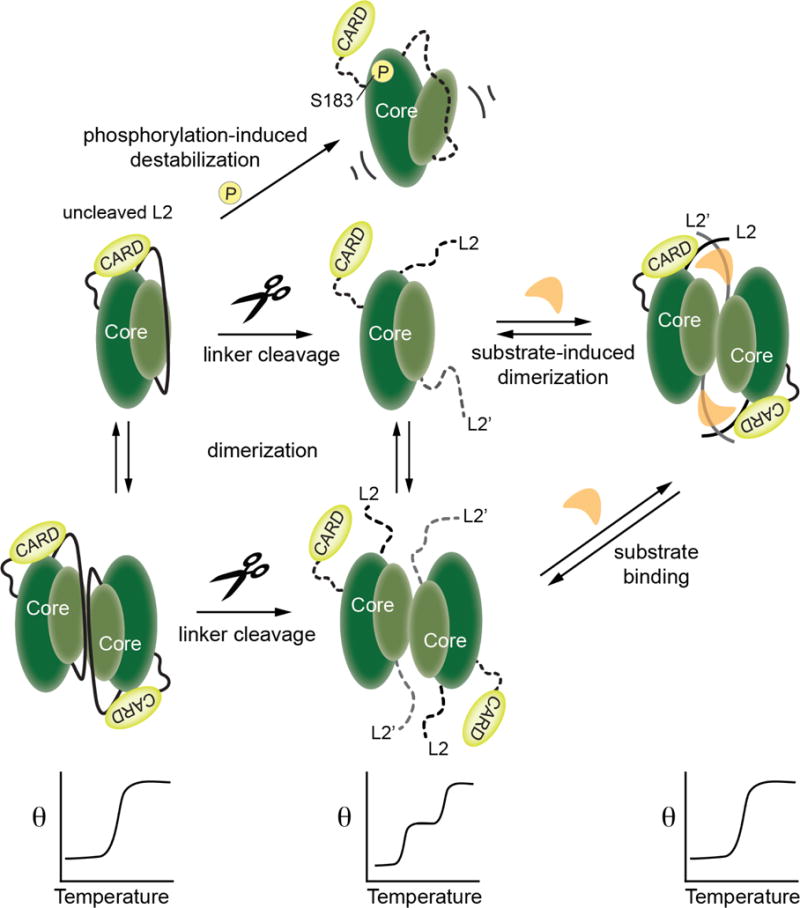
Relevant conformations of caspase-9 are shown as cartoons in the upper panels. When caspase-9 is in the uncleaved state the length of the intact, uncleaved intersubunit linker promotes the ordered conformation of the active site loops and thus facilitates interactions of the CARD with the caspase-9 core. A similar conformation is attained in a cleaved, dimeric caspase-9 when substrate binding promotes an ordered active site and thus supports the interaction of CARD with the core of the caspase-9. These CARD-core interactions result in cooperative unfolding of the two domains, as depicted in the lower panels. Caspase-9 assumes a disordered active-site conformation which abolishes the CARD-core interaction, either by transitioning to a cleaved monomeric or dimeric state in the absence of substrate, or by introducing a mutation in the core (as in S183E) that leads to its destabilization and disorder of active-site loops.
