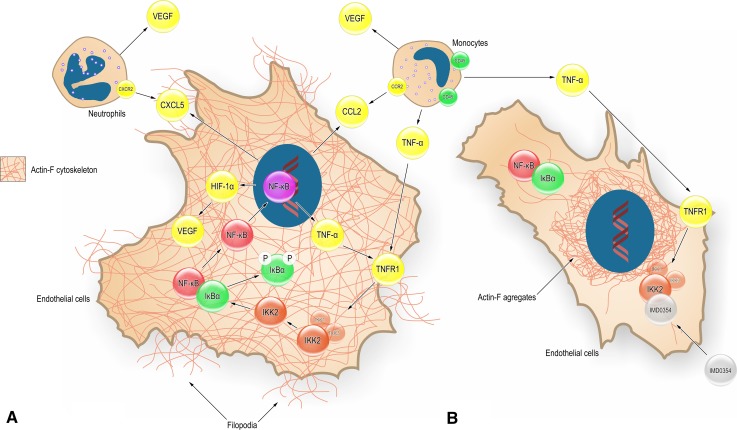
Fig. 10.
Conceptual summary of proposed cellular pathways affected by specific NF-κB blockade in the context of inflammation and angiogenesis, based on the current findings. a Endothelial cell (EC) is exposed to inflammatory stimulus TNF-α, which binds to TNFR1 and triggers a signal transduction resulting in phosphorylation of IκBα by IKK2; IκBα is ubiquitinated and degraded; this process enables the nuclear translocation of NF-κB. Upon nuclear translocation, NF-κB up-regulates a variety of pro-inflammatory and pro-angiogenic genes, including TNF-α, CXCL5, CCL2, and HIF-1α. TNF-α has auto- and paracrine effects and activates NF-kB through positive feedback, which amplifies the inflammatory response. CCL2 and CXCL5 have chemotactic effects on monocytes and neutrophils that in turn secrete a variety of cytokines, including TNF-α and VEGF. Nuclear NF-κB also up-regulates HIF-1α and by this mechanism can directly increase VEGF production. VEGF, in turn, acts on the EC, affecting actin polymerization, cytoskeleton composition, cell motility, tube formation and sprouting angiogenesis. b Selective IKK2 inhibition by IMD-0354 inhibits IκBα phosphorylation by IKK2 disrupting NF-κB activation and nuclear translocation. The pro-inflammatory and pro-angiogenic cytokine and chemokine production are substantially diminished. With decreased levels of VEGF, actin cytoskeleton formation, EC motility and migration are all suppressed. The inhibition of NF-kB also reduces the inflammatory response through suppression of the transcription of pro-inflammatory genes