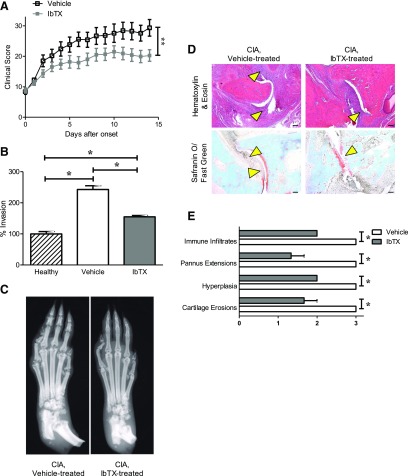
Fig. 5.
IbTX reduces disease severity in the CIA rat model of RA. (A) Clinical scores of paw inflammation of rats with CIA treated with vehicle (black) or 0.5 mg/kg IbTX (gray) every other day starting at disease onset. Data are the mean ± S.E.M. (n = 12 rats per group). (B) Ex vivo invasiveness of FLSs from three healthy Lewis rats, three rats with CIA treated with vehicle, and three rats with CIA treated with 0.5 mg/kg IbTX through Matrigel-coated Transwell inserts. Data are the mean ± S.E.M. (n = 3 per group). (C) Example X-ray images of paws from rats with CIA treated every other day with vehicle (left) or IbTX (right) for 14 days after disease onset. (D) Hematoxylin/eosin staining (top) and safranin O/fast green staining (bottom) of tissue sections of paws from rats with CIA treated with vehicle (left) or IbTX (right). Arrowheads indicate areas of hyperplasia (hematoxylin/eosin) and cartilage erosions (safranin O/fast green). (E) Histology scoring of paw joints of rats with CIA treated with vehicle (white) or IbTX (gray). Data are the mean ± S.E.M. (n = 3 paws per group). *P < 0.05; **P < 0.01. Scale bar, 100 μm in (D).