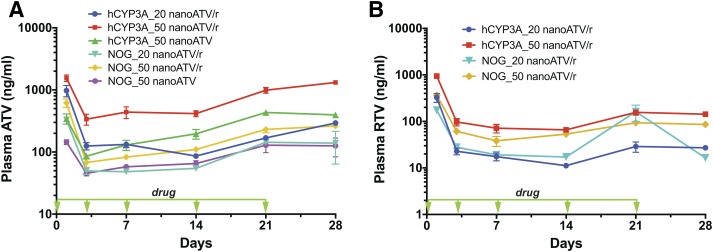
Fig. 2.
Drug plasma concentrations in nanoATV or nanoATV/r-treated mice. (A) ATV plasma concentrations in humanized hCYP3A-NOG (hCYP3A) or NOG mice treated intramuscularly with 20 or 50 mg/kg nanoATV/r or 50 mg/kg nanoATV on days 0, 3, 7, 14, and 21. (B) RTV plasma concentrations in hCYP3A and NOG mice treated with 20 or 50 mg/kg nanoATV/r. Data are expressed as mean ± S.E.M. N = 5–8.