Abstract
Background and Objectives
Primary surgery is the preferred treatment of T1–T4a sinonasal squamous cell carcinoma (SNSCC).
Methods
Patients with SNSCC in the National Cancer Data Base (NCDB) were analyzed. Factors that contributed to selecting primary surgical treatment were examined. Overall survival (OS) in surgical patients was analyzed.
Results
4,770 patients with SNSCC were included. In T1–T4a tumors, lymph node metastases, maxillary sinus location, and treatment at high-volume centers were associated with selecting primary surgery. When primary surgery was utilized, tumor factors and positive margin guided worse OS. Adjuvant therapy improved OS in positive margin resection and advanced T stage cases.
Conclusions
Tumor and non-tumor factors are associated with selecting surgery for the treatment of SNSCC. When surgery is selected, tumor factors drive OS. Negative margin resection should be the goal of a primary surgical approach. When a positive margin resection ensues, adjuvant therapy may improve OS.
Keywords: sinonasal squamous cell carcinoma (SNSCC), maxillary sinus, margin status, treatment selection, adjuvant therapy
INTRODUCTION
Overall survival (OS) of sinonasal malignancies comprise less than 5% of all head and neck tumors,[1,2] making analysis of trends in therapeutic management and adherence to treatment guidelines challenging. Among all subsites of the sinonasal tract, squamous cell carcinoma (SCC) is the most common histologic variant, with a reported incidence of more than 50% among all nasal and paranasal sinus tumors.[2-4] The maxillary sinus has been reported to be the most common site of sinonasal squamous cell carcinoma (SNSCC), followed by the nasal cavity.[1,5] SNSCC follows an insidious growth pattern and often presents with nonspecific symptoms at a locally advanced stage with involvement of adjacent critical structures such as the skull base, orbit, dura mater and brain.[1,2,5,6]
Treatment approaches for SNSCC seem to vary widely. Practice guidelines published by the National Comprehensive Cancer Network (NCCN)[7] recommend surgery as the “preferred” primary treatment for T1–T4a tumors of the ethmoid sinus and the solitary primary treatment option for T1–T4a maxillary sinus tumors. The NCCN states that the tumor should be considered surgically curable by appropriate resection, with the overarching goal of complete resection with tumor-free margins. Adjuvant radiotherapy (RT) is recommended for most tumors of the ethmoid sinus, with the option for observation in T1, negative margin, centrally located tumors, and with consideration for adjuvant chemoradiotherapy (CRT) for tumors with high-risk features. In T1–T2 negative margin resection maxillary sinus tumors without high-risk features, observation can be utilized. However, for tumors with adverse features include including high T stage (T3–T4a), adjuvant RT or consideration for CRT is recommended. CRT is recommended for positive margin resections. It remains unclear if institutions are following these guidelines, as the goals of surgery may include not only negative margin resection but also palliation, debulking prior to adjuvant CRT,[8] and reduction of radiation fields to exclude vital structures.[9] This study attempts to fill the knowledge gaps regarding treatment approaches for SNSCC over the past 15 years, the clinical and institution-related factors associated with the decision to treat SNSCC utilizing a primary surgical approach, and the variables correlated with improved OS.
MATERIALS AND METHODS
Data Source
The National Cancer Data Base (NCDB), jointly sponsored by the American Cancer Society and the American College of Surgeons, consists of a large sample of cancer cases gathered from over 1,500 hospital-based cancer registries within the United States. The source files for this study were used in accordance with the NCDB Participant Use Data File agreement.
Study Cohort
We identified all patients with SNSCC who were diagnosed from 2003 through 2012 and were ≥ 18 years of age. We included International Classification of Diseases for Oncology (ICD-O) codes for the nasal cavity, maxillary, and ethmoid. Our inclusion criteria required histologically proven SCC tissue obtained from biopsy or surgical pathology specimens and examined by microscope. We excluded patients with SCC of the nasopharynx, the middle ear, frontal, sphenoid, an unspecified accessory sinus, and overlapping lesions of accessory sinuses. We also excluded patients for whom the following variables were unknown: TNM staging, any T4 stage tumors that were not sub-classified as T4a or T4b, hospital type, hospital volume, comorbidities, surgery code, and insurance type. Additionally, any patients who received any part of their treatment outside of the NCDB-reporting facility were excluded to be certain of the exact treatment regimen administered. A total of 4,770 patients fulfilled the specified inclusion criteria.
Outcomes
The main outcome of this study was the choice of therapeutic strategy: surgical versus non-surgical treatment (RT and/or CRT). The surgical subgroup included patients undergoing surgical resection alone as well as those who may have received RT and/or CT in addition to surgery. Local tumor excision, such as polypectomy, was not considered sufficient to categorize the treatment strategy as surgical. A secondary outcome for this study was the identification of patient, tumor, treatment, and institution-related factors associated with improved OS for patients treated with primary surgery.
Covariates
Overall T (pathologic or clinical) stages were used in this study to investigate a potential association between disease stage and choice of therapeutic strategy. The patient sociodemographic factors analyzed in this study included: sex age, race, insurance status (private, government, and uninsured), and Charlson-Deyo comorbidity score. Treatment facility type was defined as either academic, which included all academic programs as well as National Cancer Institute Comprehensive Cancer Centers (NCI CCC), or as community, which included community cancer programs and comprehensive community cancer programs. Hospital volume was defined as the total number of new SNSCC cases treated overall from 2003 to 2012, with all hospitals divided into fewer than and more than 50 cases for statistical analysis. OS was defined as the number of months between the date of diagnosis and the date on which the patient was last contacted or died.
Statistical Analysis
All analyses were conducted using Stata statistical software (release 12.1; StatCorp LP, College Station, TX). This study utilized multivariable logistic regression compare patient, tumor, and treatment center-specific factors that contributed to selecting surgery as the primary treatment modality for SNSCC. A secondary analysis of factors that contributed to improve OS was performed utilizing multivariable Cox proportional hazard regression and Kaplan-Meier statistics. P values < 0.05 were considered statistically significant.
RESULTS
Patient, Tumor, and Treatment Center Characteristics
Overall, 4,770 patients with SNSCC from 2003 to 2012 met the inclusion criteria in Table 1. Three-thousand and fifty-two (64%) patients were male and 4,102 (86%) patients were white. Forty-seven percent of patients (2,241) were under the age of 65, and 3,816 (80%) patients had a Charlson-Deyo score of 0. Two-thousand six-hundred and eighty-two patients (60 %) were covered by government insurance. Two-thousand four-hundred and thirty-two patients (51%) were treated at academic or NCI CCC facilities. Low-volume hospitals that saw ≤ 50 patients with SNSCC in total from 2003 to 2012 treated 3,577 (75%) of patients. The most common tumor site was the nasal cavity for 2,528 patients (53%), followed by the maxillary sinus for 1,955 patients (41%). Regarding the staging of SNSCC cases, the largest proportion of patients had tumors with an overall T stage of T1 (1,478 or 31%) while 763 (16%), 763 (16%), 1,192 (25%), and 572 (12%) of patients had T2, T3, T4a and T4b tumors, respectively. Lymph node metastases were reported in 334 patients (7%) and distant metastases were reported in 131 patients (3%).
Table 1.
Patient Characteristics (n = 4,770)
| Age | Number of Patients |
|---|---|
| < 50 | 583 |
| 50–64 | 1,637 |
| 65–79 | 1,775 |
| > 80 | 775 |
| Sex | |
| Male | 3,041 |
| Female | 1,729 |
| Race | |
| Caucasian | 4,109 |
| Other | 661 |
| T Stage | |
| T1 | 1,474 |
| T2 | 743 |
| T3 | 762 |
| T4a | 1,197 |
| T4b | 594 |
| N stage | |
| N0 | 4,478 |
| N1–N3 | 292 |
| M stage | |
| M0 | 4,661 |
| M1 | 109 |
| Primary site | |
| Nasal Cavity | 2,512 |
| Maxillary sinus | 1,961 |
| Ethmoid sinus | 297 |
| Hospital type | |
| Academic/NCI CCC* | 2,441 |
| Community | 2,329 |
| Hospital Volume | |
| < 50 patients | 3,596 |
| > 50 patients | 1,174 |
| Insurance | |
| Private | 1,681 |
| Government | 2,870 |
| Uninsured | 719 |
| Charlson-Deyo Comorbidity Count | |
| 0 | 3,836 |
| 1 | 700 |
| > 2 | 234 |
NCI CCC-National Cancer Institute Comprehensive Cancer Center
Variables Associated with Primary Surgical Treatment for SNSCC
Overall, 1,717 (36%) of patients with SNSCC were treated utilizing a primary surgical approach. From 2003 to 2012, the overall percentage of patients treated utilizing a surgical approach remained stable (1,807, or 37.9% in 2003 vs. 1,636, or 34.3% in 2012, P = 0.627). For cases with tumors staged T1–T4a, 1,812 patients or 38% were treated with primary surgery. There was not a significant difference in primary surgical utilization during the study period (1,850 or 38.8% in 2003 vs. 1,798 or 37.7% in 2012, P = 0.742) in this cohort. By contrast, over the 9 years studied, 136 (23%) of T4b tumors were treated with surgery, with a significant decrease in primary surgical treatment utilized over time (182 or 30.7% in 2003 vs. 62 or 10.6% in 2012, P = 0.009). The most common therapeutic strategy for surgical patients during the study time period was surgical resection alone (703 or 41%), followed closely by surgery and adjuvant RT (670 or 39%). Adjuvant radiation and chemotherapy was observed in 395, or 23% of primary surgery patients.
On multivariable analysis, several patient tumor and non-tumor factors were associated with the use of a primary surgical approach. In stage T1–T4a tumors, patients over the age of 80 years (> 80 vs. < 50: Odds Ratio (OR) 0.65, CI 0.50–0.85, P = 0.002) and non-white patients (other vs. white: OR 0.67, CI 0.54–0.82, P < 0.001) were less likely to undergo a primary surgical approach. Higher T stage also was associated with a decrease in selecting surgical treatment (T4a vs. T1: OR 0.73, CI 0.61–0.89, P = 0.001). Maxillary sinus location also predicted primary surgical treatment (maxillary sinus vs nasal cavity: OR 1.29, CI 1.10–1.52, P = 0.002). Presence of regional disease (N+ vs. N0: OR 1.50, CI 1.15–1.97, P = 0.003) was associated with increased utilization of primary surgery while metastatic disease (M+ vs. M0: OR 0.39, CI 0.21–0.73, P = 0.003) was associated with less frequent use of surgery. Carrying private insurance, a non-tumor factor, was associated with higher utilization of primary surgical treatment (private vs. uninsured: OR 1.75, CI 1.63–0.88, P <0.001). Factors associated with selecting surgery as primary treatment are summarized in Table 2.
Table 2.
Factors Associated with Selecting Primary Surgery and Overall Survival
| Factors Associated with Primary Surgery | Factors Associated with Overall Survival | ||||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | |||||
| < 50 | 1 [Reference] | 1 [Reference] | |||
| 50–64 | 0.87 (0.70–1.08) | 0.19 | 1.07 (0.79–1.44) | 0.67 | |
| 65–79 | 0.93 (0.73–1.17) | 0.52 | 1.48 (1.07–2.04) | 0.02 | |
| > 80 | 0.65 (0.50–0.85) | 0.002 | 2.71 (1.89–3.89) | <0.001 | |
| T Stage | |||||
| T1 | 1 [Reference] | 1 [Reference] | |||
| T2 | 0.69 (0.57–0.83) | <0.001 | 1.18 (0.88–1.57) | 0.28 | |
| T3 | 0.88 (0.72–1.08) | 0.23 | 1.51 (1.12–2.05) | 0.007 | |
| T4a | 0.73 (0.61–0.89) | 0.001 | 1.86 (1.42–2.45) | <0.001 | |
| T4b | N/A | 2.51 (1.76–3.57) | <0.001 | ||
| N stage | |||||
| N0 | 1 [Reference] | 1 [Reference] | |||
| N1–N3 | 1.50 (1.15–1.97) | 0.003 | 2.38 (1.84–3.09) | <0.001 | |
| M stage | |||||
| M0 | 1 [Reference] | 1 [Reference] | |||
| M1 | 0.39 (0.21–0.73) | 0.003 | 2.91 (1.56–5.45) | 0.001 | |
| Primary site | |||||
| Nasal Cavity | 1 [Reference] | 1 [Reference] | |||
| Maxillary sinus | 1.29 (1.10–1.52) | 0.002 | 1.54 (1.25–1.89) | <0.001 | |
| Ethmoid sinus | 1.12 (0.82–1.53) | 0.47 | 1.45 (1.00–2.12) | 0.052 | |
| Hospital type | |||||
| Academic/NCI CCC* | 1 [Reference] | 1 [Reference] | |||
| Community | 0.96 (0.83–1.12) | 0.64 | 0.92 (0.76–1.12) | 0.39 | |
| Race | |||||
| Caucasian | 1 [Reference] | 1 [Reference] | |||
| Other | 0.67 (0.54–0.82) | <0.001 | 1.04 (0.81–1.35) | 0.73 | |
| Hospital Volume | |||||
| < 50 patients | 1 [Reference] | 1 [Reference] | |||
| > 50 patients | 1.10 (0.93–1.31) | 0.26 | 0.89 (0.72–1.10) | 0.29 | |
| Insurance | |||||
| Private | 1 [Reference] | 1 [Reference] | |||
| Government | 0.65 (0.46–0.92) | 0.01 | 1.15 (0.72–1.81) | 0.56 | |
| Uninsured | 0.75 (0.63–0.88) | <0.001 | 1.15 (0.93–1.44) | 0.19 | |
| Sex | |||||
| Male | 1 [Reference] | 1 [Reference] | |||
| Female | 1.01 (0.89–1.16) | 0.86 | 0.84 (0.71–1.00) | 0.046 | |
| Charlson-Deyo Comorbidity Count | |||||
| 0 | 1 [Reference] | 1 [Reference] | |||
| 1 | 1.16 (0.97–1.39) | 0.11 | 1.17 (0.94–1.46) | 0.17 | |
| > 2 | 1.15 (0.86–1.54) | 0.35 | 1.46 (1.04–2.05) | 0.03 | |
| Therapy | N/A | ||||
| Surgery alone | 1 [Reference] | ||||
| Adj RT* | 0.66 (0.54–0.81) | <0.001 | |||
| Adj CRT* | 0.66 (0.51–0.86) | 0.002 | |||
| Margin | N/A | ||||
| Negative | 1 [Reference] | ||||
| Microscopic | 1.74 (1.40–2.16) | <0.001 | |||
| Macroscopic | 1.43 (1.14–1.80) | 0.002 | |||
OR- Odds Ratio; CI - Confidence Interval; Adj RT-Adjuvant Radiation Therapy; Adj CRT- Adjuvant Chemoradiation Therapy; NCI CCC- National Cancer Institute Comprehensive Cancer Center
When patients underwent primary surgery for treatment of SNSCC, 1212 or 70.6% cases achieved a negative margin resection, while 273 or 15.9% cases had microscopic residual disease with 202 (13.4%) having macroscopic positive margin resection.
In cases with negative margin resection, patients were more likely to have surgery alone (no adjuvant treatment 993 patients or 82.0%), while when macroscopic and microscopic positive margins were present patients were more likely to have adjuvant therapy (no adjuvant treatment 758 patients or 62.6%) (P <0.001).
Survival Analysis in Patients Treated with Primary Surgery
OS at 5 years was 959 patients or 55.9% in the cohort treated with primary surgery. Median follow-up of surviving patients (864) is 53.01 months (range 0–128.67) and the mean is 55.03 months. Using Kaplan-Meier statistics, T stage predicted for OS (P < 0.001) in Figure 1a. Patients with negative margin resection had improved survival when compared to either a microscopic or macroscopic positive margin resection (P < 0.001). Patients with microscopic vs. macroscopic positive margin were not significantly different in Figure 1b.
Figure 1.
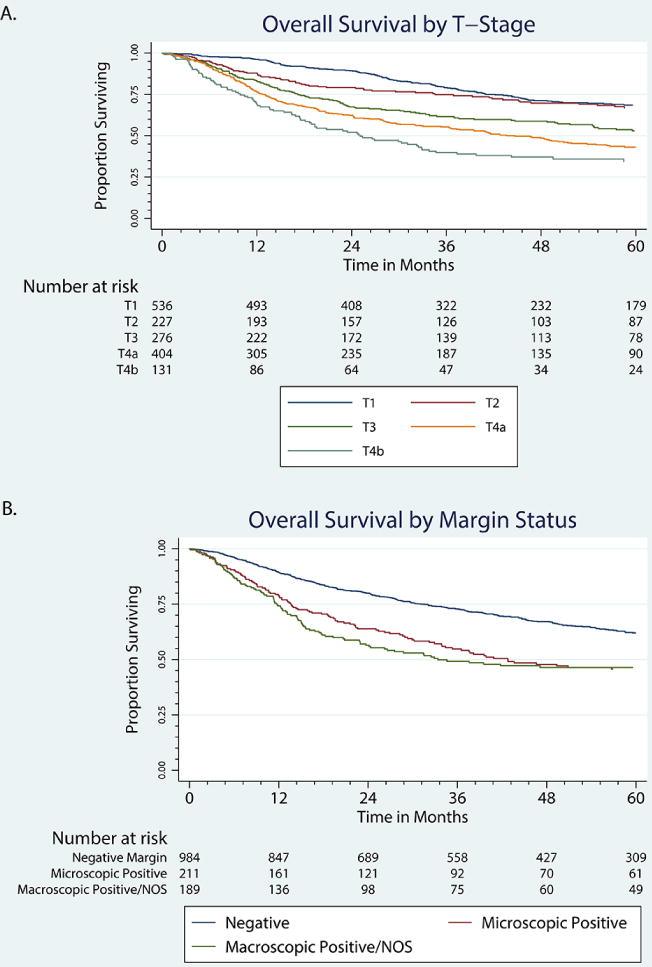
Overall survival in patients treated with primary surgery by T stage and margin status. a) T stage, b) Margin
*NOS, not otherwise specified.
On multivariate analysis, patient, tumor, and treatment factors affected survival in Table 2. Poorer survival was associated with patient factors including increased age (> 80 years old vs. < less than 50: hazard ratio (HR) 2.71, CI 1.89-3.89, P < 0.001) and increased Charlson-Deyo comorbidity score (>2 vs. 0: HR 1.46, CI 1.04–2.05, P = 0.03). Tumor factors including advanced T stage (T3 vs. T1: HR 1.51, CI 1.12–2.05, P = 0.007; T4a vs. T1: HR 1.86, CI 1.42–2.45, P < 0.001; T4b vs. T1: HR 2.51, CI 1.76–3.57, P < 0.001) nodal disease: N+ vs. N0: HR 2.38, CI 1.84–3.09, P < 0.001), distant metastases (M+ vs. M0: HR 2.91, CI 1.56–5.45, P = 0.001), and maxillary sinus location (maxillary sinus vs. nasal cavity: HR 1.54, CI 1.25–1.89, P < 0.001) were associated with worse survival. Non-tumor factors, including insurance status and medical center type, did not affect survival. Treatment factors, including a positive margin resection (microscopic margin vs. negative margin: OR 1.74, CI 1.40–2.16, P < 0.001; macroscopic margin vs. negative margin: OR 1.43, CI 1.14–1.80, P = 0.002), were associated with worse survival, while use of adjuvant therapy (surgery + RT vs. surgery alone: HR 0.66, CI 0.54–0.81, P < 0.001; surgery + CRT vs. surgery alone: HR 0.66, CI 0.51–0.86, P = 0.002) improved survival.
To assess if adjuvant therapy improved survival across all T stages, survival by T stage was analyzed independently. When controlled for patient and tumor factors, adjuvant therapy had survival benefit across margin status in Table 3. In patients with positive margin resection, adjuvant RT and CRT (surgery + RT vs. surgery alone: HR 0.48, CI 0.35–0.68, P <0.001; surgery + CRT vs. surgery alone: HR 0.44, CI 0.30–0.65, P < 0.001) were both associated with a significant improvement in survival. This benefit was not observed in patient with a negative margin resection.
Table 3.
Margin control predictor of survival
| Negative Margin | Positive Margin | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | ||||
| < 65 | 1 [Reference] | 1 [Reference] | ||
| > 65 | 1.88 (1.51–2.32) | <0.001 | 1.66(1.26–2.18) | <0.001 |
| T Stage | ||||
| T1 | 1 [Reference] | 1 [Reference] | ||
| T2 | 1.16 (0.83–1.62) | 0.39 | 1.65 (0.96–2.84) | 0.07 |
| T3 | 1.58 (1.14–2.20) | 0.006 | 2.72 (1.69–4.36) | <0.001 |
| T4a | 2.13 (1.59–2.84) | <0.001 | 2.85 (1.87–4.35) | <0.001 |
| T4b | 3.52 (2.31–5.39) | <0.001 | 3.61 (2.18–5.95) | <0.001 |
| N stage | ||||
| N0 | 1 [Reference] | 1 [Reference] | ||
| N1–N3 | 2.47 (1.74–3.51) | <0.001 | 2.41 (1.61–3.58) | <0.001 |
| Charlson-Deyo Comorbidity Count | ||||
| 0 | 1 [Reference] | 1 [Reference] | ||
| 1 | 1.16 (0.87–1.55) | 0.31 | 1.23 (0.87–1.73) | 0.87 |
| > 2 | 1.63 (1.05–2.53) | 0.03 | 1.06 (0.62–1.80) | 0.62 |
| Therapy | ||||
| Surgery alone | 1 [Reference] | 1 [Reference] | ||
| Adj RT* | 0.80 (0.63–1.02) | 0.07 | 0.48 (0.35–0.68) | <0.001 |
| Adj CRT* | 0.93 (0.66–1.30) | 0.66 | 0.44 (0.30–0.65) | <0.001 |
OR- Odds Ratio; CI- Confidence Interval; Adj RT-Adjuvant Radiation Therapy; Adj CRT- Adjuvant Chemoradiation Therapy
Using Kaplan-Meier statistics, the effects of adjuvant therapy in survival were analyzed by T stage. In patients with T1–T3 tumors, there was no significant difference in survival based on adjuvant therapy (P = 0.1404) in Figure 2a. T4a tumors had a significant survival advantage with adjuvant RT therapy and CRT compared to surgery alone. (P = 0.0008 and 0.04, respectively) in Figure 2b. T4b tumors had a significant survival advantage with adjuvant CRT and RT therapy compared to surgery only (P <0.0001 and P = 0.003, respectively) in Figure 2c.
Figure 2.
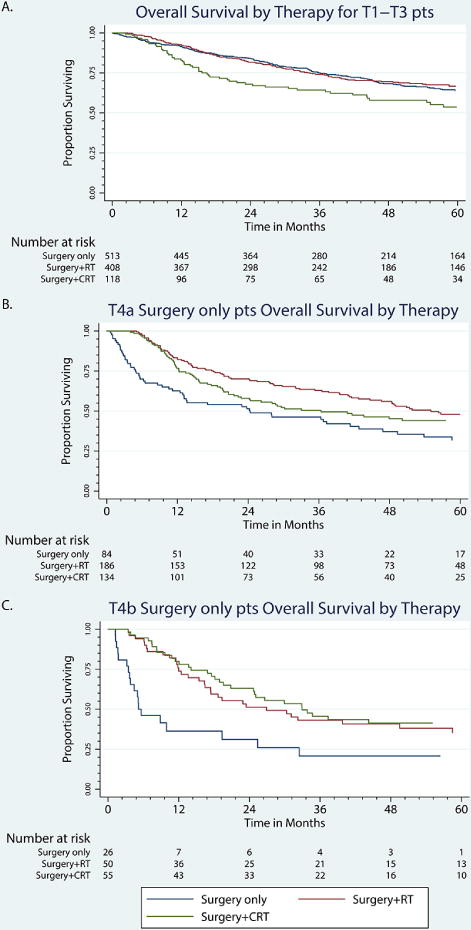
Overall survival in patients with primary surgery undergoing adjuvant RT or with adjuvant CRT by tumor stage. a) T1-T3, b) T4a, c) T4b.
*RT, radiotherapy; CT, chemoradiotherapy.
On multivariable analysis, while controlling for age, comorbidities, N stage, and margin, survival benefits associated with the addition of adjuvant radiation were only observed in T4a tumors (surgery + RT vs. surgery alone: HR 0.65, CI 0.45–0.93, P = 0.02). In T4b tumors, survival benefits were observed in patients treated with surgery and adjuvant chemoradiation (surgery + CRT vs. surgery alone: HR 0.47, CI 0.24–0.93, P = 0.03). No benefit of adjuvant therapy was observed in T1–T3 tumors in Table 4.
Table 4.
Benefits of Adjuvant Therapy by T stage*
| OR (95% CI) | P-value | |
|---|---|---|
| T1 | ||
| Surgery alone | 1 [Reference] | |
| Adj RT** | 0.99 (0.68–1.44) | 0.96 |
| Adj CRT** | 2.03 (0.64–6.46) | 0.23 |
| T2 | ||
| Surgery alone | 1 [Reference] | |
| Adj RT | 0.62 (0.37–1.03) | 0.07 |
| Adj CRT | 0.75 (0.36–1.54) | 0.44 |
| T3 | ||
| Surgery alone | 1 [Reference] | |
| Adj RT | 0.68 (0.43–1.09) | 0.11 |
| Adj CRT | 0.56 (0.31–1.02) | 0.06 |
| T4a | ||
| Surgery alone | 1 [Reference] | |
| Adj RT | 0.65 (0.45–0.93) | 0.02 |
| Adj CRT | 0.73 (0.49–1.08) | 0.11 |
| T4b | ||
| Surgery alone | 1 [Reference] | |
| Adj RT | 0.68 (0.33–1.37) | 0.28 |
| Adj CRT | 0.47 (0.24–0.93) | 0.03 |
When controlled for age, n stage, margin status, and comorbidity
OR, Odds Ratio; CI-Confidence Interval; Adj RT-Adjuvant Radiation Therapy; Adj CRT-Adjuvant Chemoradiation Therapy
DISCUSSION
While SNSCC represents the most common histology observed in the sinonasal tract, the rare nature of this pathologic entity makes analysis of trends in therapeutic management, adherence to treatment recommendations, and benefits of treatment difficult to assess. Primary surgery with adjuvant therapy (RT or CRT) represents the preferred treatment approach for sinonasal cancers stages T1–T4a. NCCN guidelines recommend treatment of “unresectable with negative margin” tumors, including T4b or very advanced local disease (tumors invading the orbital apex, cranial nerves, clivus, nasopharynx, brain, and dura) with radiation-based approaches with concurrent chemotherapy or accrual to clinical trials. Over the past 15 years, the introduction of endoscopic resection of sinonasal tumors has enabled enhanced visualization and greater margin control, with consequent decreased morbidity.
Despite advances in surgical technology during the period studied, our analysis shows that the utilization of a primary surgical approach has remained stable. While a discussion of endoscopic vs. open approaches is beyond the scope of the current work, the observed stability may represent hesitation either to adopt endoscopic approaches for malignant disease or lack of access to surgical endoscopy platforms… Independent of approach, primary surgical resection for T1–T4a was not the primary treatment for SNSCC nationally despite NCCN guideline recommendations. The current work identifies multiple patient, tumor, and non-tumor factors that attribute to deviation from the recommend primary surgical approach. Advanced T stage predicted for non-surgical approaches while in contrast, N+ disease predicted for primary surgical options. As anticipated, patients with advanced age and co-morbidities were less likely to undergo surgery. Interestingly, patients with private insurance were more likely to have primary surgery. Patients with locally advanced T4b tumors had surgery in one-quarter of cases. Surgery in this locally advanced setting may indicate comfort in resection of dura or brain in the hopes of achieving negative margin; however, this finding underscores the national deviation from guideline recommendations.
When primary surgery is selected, patient, tumor, and treatment factors determine OS. Consistent with previous reports, advanced age and comorbidities predict for worse OS.[10] Whether this decrease in survival is related to a decrease in primary surgery in these patients is unclear. In addition, lower T stage without metastatic disease was associated with a significant improvement in survival. While tumor factors are intrinsic, important, potentially alterable treatment factors emerged as important variables in predicting survival. Negative margin resection was associated with significant improvement in OS compared to both microscopic and macroscopic positive margin resection. This finding supports the importance of a negative margin resection and provides evidence to argue against “debulking” surgical interventions. This has been shown to be critically important in retrospective studies for both open and endoscopic approaches.[11,12]
Adjuvant therapy was also associated with survival differences. In SCCs of the head and neck, including SNSCC,[13] adjuvant RT has been adopted due to its local control benefits. The studies most influential in clarifying the role of the addition of postoperative chemotherapy to radiation in high risk patients with head and neck SCC are the European Organization for Research and Treatment of Cancer (EORTC) 22931 and Radiation Therapy Oncology Group (RTOG) 9501, which did not include patients with paranasal sinus malignancies. These two multicenter randomized control trials demonstrated increased locoregional control and disease-free survival for patients receiving postoperative chemoradiation compared to radiation alone and subsequently altered the standard of postoperative care of patients with head and neck SCCs. OS was not significantly different in the RTOG group but was in the EORTC group (EORTC 22931[14] and RTOG 9501[15]).
Interestingly, in the current analysis, some patients who received adjuvant therapy had a survival benefit. Specifically, adjuvant therapy benefits were observed in patients with positive margin resection (gross and microscopic margins), providing some support for extrapolation of the findings in EORTC 22931 and RTOG 9501 to SNSCC. Importantly, benefits of adjuvant therapy were not observed in T1–T3 tumors; however, patients withT4a tumors benefited from adjuvant RT, and patients withT4b tumors benefited from adjuvant CRT. These findings suggest that some patients with surgically treated tumors benefit from adjuvant therapy, including some patients with locally advanced tumors (T4a and T4b) and positive margin resection, independent of macroscopic or microscopic margin status. It is important, however, to realize these data were not produced in a trial setting and cannot be used in setting treatment guidelines.
This analysis has several limitations. The completeness and accuracy of information reported to the NCDB could not be verified and is dependent on self-auditing by each reporting center. Potential errors in reporting or selecting reporting may thus affect our results. Additionally, a number of SNSCC cases were potentially excluded due to exclusion of overlapping lesions in the nasopharynx or of the accessory sinuses. Third, although we did not consider “local tumor excision” or “polypectomy” to be surgical treatment approaches, it is possible that such patients did in fact undergo surgery for oncologic therapy or those others that were included in the surgical subgroup, did not in fact undergo primary surgical resection. In addition, we removed patients without complete data sets, which could potentially bias results.
CONCLUSIONS
Primary surgical resection is utilized in the minority of patients with SNSCC and its utilization in this patient population has remained stable over time. Tumor and non-tumor factors are associated with treatment selection. In patients undergoing primary surgical treatment, survival is associated with static tumor variables and potentially alterable treatment factors, including negative margin resection. Tumors with advanced T stage and positive margin resection benefit from adjuvant therapy. The current work suggests significant, nationwide deviation from NCCN guidelines for treatment of SNSCC.
Summary.
Primary surgery is the preferred treatment of sinonasal squamous cell carcinoma (SNSCC). When surgery is selected, tumor factors drive overall survival. Negative margin resection should be the goal of a primary surgical approach. When a positive margin resection ensues, adjuvant therapy may improve overall survival.
Acknowledgments
Funding support: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviation List
- SNCC
sinonasal squamous cell carcinoma
- NCDB
National Cancer Database
- OS
Overall Survival
- SCC
squamous cell carcinoma
- NCCN
National Comprehensive Cancer Network
- RT
radiotherapy
- CRT
chemoradiotherapy
- ICD-O
International Classification of Diseases for Oncology
- NCI CCC
National Cancer Institute Comprehensive Cancer Center
- OR
Odds Ratio
- CI
Confidence Interval
- HR
Hazard Ratio
- EORTC
European Organization for the Research and Treatment of Cancer
- RTOG
Radiation Therapy Oncology Group
Footnotes
Conflict of interest disclosures: None to report
References
- 1.Sanghvi S, Khan MN, Patel NR, et al. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124:76–83. doi: 10.1002/lary.24264. [DOI] [PubMed] [Google Scholar]
- 2.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 3.Dulguerov P, Jacobsen MS, Allal AS, et al. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–3029. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Dutta R, Dubal PM, Svider PF, et al. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125:2491–2497. doi: 10.1002/lary.25465. [DOI] [PubMed] [Google Scholar]
- 5.Michel J, Fakhry N, Mancini J, et al. Sinonasal squamous cell carcinomas: clinical outcomes and predictive factors. Int J Oral Maxillofac Surg. 2014;43:1–6. doi: 10.1016/j.ijom.2013.07.741. [DOI] [PubMed] [Google Scholar]
- 6.Kermer C, Poeschl PW, Wutzl A, et al. Surgical treatment of squamous cell carcinoma of the maxilla and nasal sinuses. J Oral Maxillofac Surg. 2008;66:2449–2453. doi: 10.1016/j.joms.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Song CM, Won TB, Lee CH, et al. Treatment modalities and outcomes of olfactory neuroblastoma. Laryngoscope. 2012;122:2389–2395. doi: 10.1002/lary.23641. [DOI] [PubMed] [Google Scholar]
- 8.Hanna E, DeMonte F, Ibrahim S, et al. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009;135:1219–1224. doi: 10.1001/archoto.2009.173. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida JR, Su SY, Koutourousiou M, et al. Endonasal endoscopic surgery for squamous cell carcinoma of the sinonasal cavities and skull base: Oncologic outcomes based on treatment strategy and tumor etiology. Head Neck. 2015;37:1163–1169. doi: 10.1002/hed.23731. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Hanai N, Nishikawa D, et al. The Charlson comorbidity index is a prognostic factor in sinonasal tract squamous cell carcinoma. Jpn J Clin Oncol. 2016;46:646–651. doi: 10.1093/jjco/hyw049. [DOI] [PubMed] [Google Scholar]
- 11.Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck. 2005;27:575–584. doi: 10.1002/hed.20165. [DOI] [PubMed] [Google Scholar]
- 12.Batra PS, Luong A, Kanowitz SJ, et al. Outcomes of minimally invasive endoscopic resection of anterior skull base neoplasms. Laryngoscope. 2010;120:9–16. doi: 10.1002/lary.20680. [DOI] [PubMed] [Google Scholar]
- 13.Bentz BG, Bilsky MH, Shah JP, Kraus D. Anterior skull base surgery for malignant tumors: a multivariate analysis of 27 years of experience. Head Neck. 2003;25:515–520. doi: 10.1002/hed.10250. [DOI] [PubMed] [Google Scholar]
- 14.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]


