Abstract
Large portions promote intake of energy dense foods (i.e., the portion size effect--PSE), but the neurobiological drivers of this effect are not known. We tested the association between blood oxygen level dependent (BOLD) brain response to food images varied by portion size (PS) and energy density (ED) and children’s intake at test-meals of high- and low-ED foods served at varying portions. Children (N = 47; age 7–10 years) participated in a within-subjects, crossover study consisting of 4 meals of increasing PS of high- and low-ED foods and 1 fMRI to evaluate food images at 2 levels of PS (Large, Small) and 2 levels of ED (High, Low). Contrast values between PS conditions (e.g., Large PS - Small PS) were calculated from BOLD signal in brain regions implicated in cognitive control and reward and input as covariates in mixed models to determine if they moderated the PSE curve. Results showed a significant effect of PS on intake. Responses to Large relative to Small PS in brain regions implicated in salience (e.g., ventromedial prefrontal cortex and orbitofrontal cortex) were positively associated with the linear slope (i.e., increase in intake from baseline) of the PSE curve, but negatively associated with the quadratic coefficient for the total meal. Responses to Large PS High ED relative to Small PS High ED cues in regions associated with cognitive control (e.g., dorsolateral prefrontal cortex) were negatively associated with the linear slope of the PSE curve for high-ED foods. Brain responses to PS cues were associated with individual differences in children’s susceptibility to overeating from large portions. Responses in food salience regions positively associated with PSE susceptibility while activation in control regions negatively associated with PSE susceptibility.
Keywords: portion size, pediatrics, fMRI, eating behavior, brain
Introduction
Portion size (PS) has a robust effect on intake in both adults (Kral & Rolls, 2004; Roe, Kling, & Rolls, 2016; Rolls, Morris, & Roe, 2002; Rolls, Roe, & Meengs, 2007) and children (Fisher, 2007; Fisher, Arreola, Birch, & Rolls, 2007; Fisher, Liu, Birch, & Rolls, 2007; Kral, Kabay, Roe, & Rolls, 2010; Mathias et al., 2012; Mooreville et al., 2015). This portion size effect (PSE) has been observed across individual variations in weight status (Brunstrom, Rogers, Pothos, Calitri, & Tapper, 2008; Fisher, Arreola, Birch, & Rolls, 2007), dietary restraint (Rolls, Morris, & Roe, 2002), and age (Fisher, 2007). However, recent evidence suggests a relationship between the PSE and appetitive traits (e.g., food responsiveness, satiety responsiveness) linked to overeating in children (Kling, Roe, Keller, & Rolls, 2016; Mooreville et al., 2015). Therefore, increased susceptibility to the PSE presents a risk factor for obesity, especially considering the widespread availability of large portions for many common energy dense entrées, snacks, and beverages (Nielsen & Popkin, 2003; Young & Nestle, 2003). Currently, the mechanisms underlying the PSE are not well understood (English, Lasschuijt, & Keller, 2015). The purpose of this study was to identify brain regions associated with the PSE by determining the relationship between brain responses to food images varied by PS and energy density (ED) and laboratory intake from multi-item test-meals of high- and low-ED foods served at varying portions.
Blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) has been used to characterize individual differences in brain response to the presentation of food cues. Studies using BOLD fMRI have identified differences in brain processing as a function of food energy content (Frank et al., 2010; Killgore, Young, Femia, & Bogorodzki, 2003; Killgore & Yurgelun-Todd, 2005; Stoeckel et al., 2008), appetitive state (i.e., fed vs. fasted) (Dimitropoulos, Tkach, Ho, & Kennedy, 2012; Frank et al., 2010; Goldstone et al., 2009; LaBar et al., 2001), and body weight (Batterink, Yokum, & Stice, 2010; Bruce et al., 2010; Carnell, Benson, Pantazatos, Hirsch, & Geliebter, 2014; Davids et al., 2010; Martin et al., 2009; Stoeckel et al., 2008). We previously reported that brain regions implicated in cognitive control and goal directed behavior (i.e., inferior frontal gyrus-IFG) are activated in response to PS cues, while energy-density (ED) activates a broader network of regions implicated in appetite, emotion, and reward (e.g., caudate and anterior insula) (English et al., 2016; English et al., 2017). An unanswered question is how variations in brain response to food PS cues relate to children’s energy intake from increasing portions of foods served at a meal.
The current study is part of a larger investigation aimed at identifying brain regions associated with portion size response in children. We developed our hypotheses based on the notion that individuals who find larger portions more rewarding might be more susceptible to overeating in their presence, while those who are able to successfully activate self-control mechanisms when presented with large portions should be less susceptible to overeating in their presence. If this is true, we should find a positive association between increased susceptibility to overeating from large portions and brain activation induced by large portion food cues in regions involved in drive, motivation, and reward. Alternatively, we should find a negative association between the susceptibility to overeat from large portions and food cue-induced activation in brain regions implicated in cognitive control. Testing the association between brain response to visual PS cues and intake from test-meals where portion sizes are increased will allow us to disentangle the effects of different brain systems on the PSE, and in the future, this information could be applied to the design of tailored interventions to help consumers moderate overeating.
Therefore, in the present investigation, we hypothesize that children who have increased brain response to large relative to small PS food cues in brain regions implicated in reward and appetitive value will show increased susceptibility to the PSE in the laboratory. Second, we hypothesize that children who have increased brain responses to large relative to small PS food cues in brain regions implicated in cognitive control will show reduced susceptibility to the PSE in the laboratory. Additionally, PS and ED have independent and additive effects on energy intake, with the greatest intake coming from large portions of energy dense entrées (Kling, Roe, Keller, & Rolls, 2016; Kral & Rolls, 2004). Therefore, we conduct secondary analyses to identify brain regions associated with the PSE for high-ED and low-ED foods, analyzed separately. Furthermore, to explore the extent to which the relationships between brain activation and laboratory food intake may reflect habitual consumption patterns, we report associations between brain activation to PS cues and child weight status.
Methods
Study Design
The study used a within-subjects, repeated measures crossover design where children completed 4 test-meal visits and 1 fMRI scan on the 5th visit. The order of test-meal visits was randomized, and order sequence was counter-balanced across children. The fMRI was always done on the 5th visit to allow children time to acclimate to the study staff and to accommodate two mock training sessions completed after study visits 3 and 4. Baseline assessments (i.e., eating behavior, demographic, and usual feeding practices) and anthropometrics were completed on the first visit. Within families, all visits were conducted at the same time of day, either lunch or dinner, depending on availability. Time of day tested was accounted for in statistical analyses, but did not influence the main outcomes. Data collection was completed over July 2013 – July 2015 and the study was approved by the Institutional Review Board of The Pennsylvania State University.
We previously published results from a whole-brain fMRI analysis of the first 36 children tested in this study, prior to an update in the scanner equipment (English et al., 2017). The scanner update improved the resolution of the images, so we limited the whole brain analyses to the 36 children tested on the same equipment. In the present study, results are reported for the entire cohort from analyses that include signal-to-noise ratio (SNR) and temporal SNR (TSNR) as covariates to adjust for differences in scanner resolution. A comparison of results from the two studies is provided in the discussion.
Participants and Sample Size Determination
In order to participate, children had to be healthy, without food allergies, not currently taking medications that might influence appetite or brain response, and right handed to eliminate bias due to brain hemisphere dominance (Rasmussen & Milner, 1977). A total of 77 families were screened to enroll a cohort of 54 children in the entire study. We excluded children who had metal implants or dental work and diagnosed psychological conditions (e.g., claustrophobia, anxiety, and attention disorders) that might impact fMRI scan success. Of the 54 children enrolled, 4 did not participate in the fMRI for various reasons (e.g., undisclosed metal retainer, loss to follow-up). Of the 50 children who completed the MRI, 3 had excessive motion in the scanner and were excluded from analysis, leaving a final sample of 47 children for the present study (94% success rate). Parents provided written consent for their child’s participation and children provided oral and written assent. Descriptive data on these 47 children are displayed in Table 1.
Table 1.
Characteristics of the 47 children with complete data
| Continuous Variables* | Range | Mean ± SD |
|---|---|---|
|
| ||
| Age (years) | 7.2 – 10.9 | 8.9 ± 1.2 |
|
| ||
| BMI z-score | −1.5 – 2.2 | 0.1 ± 0.9 |
|
| ||
| BMI-for-age % | 6.1 – 97.9 | 52.7 ± 28.4 |
|
| ||
| Categorical Variables** | % (n) | |
|
| ||
| Sex | ||
| Girls | 53.2 (25) | |
| Boys | 46.8 (22) | |
|
| ||
| Ethnicity | ||
| Caucasian | 93.6 (44) | |
| Non-Caucasian | 6.4 (3) | |
N=47 for all continuous variables
N=47 for all categorical variables
As there were no prior studies testing brain response to PS and ED cues and correlating this response to objectively measured intake, we determined sample size by consulting the broader food cue literature in children (Bruce et al., 2010; Davids et al., 2010; van Meer, van der Laan, Adan, Viergever, & Smeets, 2015), to estimate expected effect sizes ranging from 0.5 – 0.65 in reward-based regions and 0.4 to 0.7 in control regions. Based on these expected effect sizes, we used G*power software to calculate that we needed 50 children to provide 80% power to detect significant outcomes. Because this was a pilot and feasibility study, we were primarily powered for the fMRI portion, but over-recruited by approximately 20%, to allow testing of our behavioral aims.
Laboratory Assessment of the PSE
Children’s intake in response to PS was assessed across 4 test-meals where the amount served of age-appropriate, widely acceptable foods was systematically increased. Because the relationship between amount served and amount consumed is curvilinear (Roe et al., 2016), 4 test-meal conditions have been found to be necessary for studying individual differences in the response to PS. The PS of the 100% reference condition (i.e., baseline) was selected to be consistent with previous studies (Leahy, Birch, & Rolls, 2008; Spill, Birch, Roe, & Rolls, 2010), and was intended to provide children with sufficient amount and variety to simulate usual consumption. The PS for all items at the meal was increased simultaneously by weight to reach three additional conditions of 137%, 167% and 200%. Foods were selected based on experience from previous studies that offered test-meals to children of similar demographics (Leahy et al., 2008; Spill et al., 2010). We also selected a variety of both high- and low-ED food options for the examination of secondary hypotheses aimed at identifying brain regions implicated in the PSE for foods differing in ED. The three low-ED foods were cherry tomatoes, red grapes, and lightly buttered broccoli (ED ranging from 0.2 – 0.7 kcal/g). The three medium/high-ED foods were pasta and cheese, garlic bread, and angel food cake (ED ranging from 1.5 – 3.6 kcal/g). For brevity, the latter category will be referred to as high-ED from hereon. At each meal, children consumed water ad libitum as a beverage.
After the meal, leftovers were weighed to the nearest 0.1 g on a scale (Ohaus, Parsippany, NJ). Consumption was computed as the difference between pre- to post-meal weights (grams) of each food. Energy and nutrient intakes were calculated from each food from the Nutrition Facts Panel and a standard food composition database (US Department of Agriculture, 2015).
Test-meal visit procedures
Children completed 5 study sessions at lunch (11:00 am – 1:00 pm) or dinner (4:00 pm – 6:00 pm), depending on family availability. Visit time was consistent within-family, and to the extent possible, counter-balanced across families. Children attended each visit at least 2 hours fasted (to approximate fasting conditions observed prior to a meal) and completed all procedures in a one-on-one testing environment with the researcher. To assess covariates that might impact meal consumption, child fullness level was assessed before and after each meal and prior to the fMRI using an age-appropriate, pictorial visual analog scale (Keller et al., 2006). Additionally, to examine possible covariates associated with test-meal consumption, explicit liking and wanting of the test-meal foods were assessed pre- and post-meal on a 150-mm visual analog scale anchored with “not at all” and “like/want very much”. Following these measures, children were given 30 minutes to eat ad libitum from the test-meal while a researcher read a pre-approved, non-food related book to the child. On the baseline visit, parents completed questionnaires to assess usual child eating behaviors and parental feeding practices and children completed additional measures of portion size assessment (to be reported elsewhere).
Anthropometrics
Child height, weight, and body fat percentage were assessed with shoes and coats removed on a stadiometer (Seca® model 202 Chino, CA), standard scale (Detecto ® model 437, Webb City, MO) and bioelectric impedance analysis scale (Tanita® model BF-350, Arlington Heights IL). Height and weight were converted to body mass index (BMI) and BMI z-score based on the Centers for Disease Control and Prevention growth charts as the weight-to-height ratio for age and sex (Cole, Bellizzi, Flegal, & Dietz, 2000).
fMRI training
Following the test-meal on visits 3 and 4, children completed a training session in a mock (simulated) MRI environment. Full details on this training have been previously reported (English et al., 2015). The purpose of this training was to increase child comfort with the procedures and reduce motion artifact during the actual scanning session.
fMRI Stimuli and Paradigm
Images shown to children during the fMRI were created for this study and were designed to represent common, age-appropriate low-ED (< 1.5 kcal/g) and medium/high-ED (> 1.5 kcal/g) foods, presented at two levels of PS (Large, Small). We developed the fMRI stimuli to correspond with the food categories served at the test-meal (i.e., low- and high-ED foods served at varying PS). For space purposes, the ED groups will be referred to as Low-ED and High-ED herein. Food choices as well as the serving sizes shown to children were selected by consulting amounts reported for similar age children in the Continuing Survey of Food Intakes by Individuals (Smiciklas-Wright, Mitchell, Mickle, Cook, & Goldman, 2002). The Small PS was selected as the 10th percentile for the amount commonly consumed while the Large PS was the 90th percentile of amount commonly consumed. Full details on the development of these images were previously reported (English et al., 2015) and are available from the authors upon request.
During the fMRI, children viewed images in a block presentation design containing 4 food conditions (Large PS High ED, Small PS High ED, Large PS Low ED, and Small PS Low ED) and 2 control conditions (furniture and scrambled images). Across the scan, children viewed 180 total images (30 images per condition), presented in 6 functional runs. Within each run, images were shown in blocks of 5, presented in a pseudo-randomized fashion so that a child would not see more than 2 food blocks in a row before seeing a control block. Each image was presented for 2 seconds followed by a 0.5 fixation cross between images and a 2–11 second jittered interval between blocks. Total time for the scan ranged from 21 – 35 minutes, with an average time of 25 minutes.
Following the fMRI, children used the same 150-mm visual analog scale used at the test-meals to rate explicit liking and wanting for the food and control stimuli. Summary data on these measures have been reported previously(English et al., 2017).
fMRI data acquisition
The first 36 children were scanned on a Siemens 3.0 Tesla MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with a standard 12-channel head coil. Due to an update in scanner equipment that occurred during data collection, the remaining 11 children were scanned on Siemens 3.0 Tesla Magnetom Prisma Fit (Siemens Medical Solutions, Erlangen, Germany) with 20-channel head coil. The Social Life and Engineering Sciences Imaging Center conducted tests to determine impact of the scanner update on brain response to a well-validated sensory-motor task in 31 adult participants. There were no statistically significant differences in functional data obtained from the task in scans conducted pre- versus post-update. Because both the SNR and TSNR (i.e., ratio of mean signal to standard deviation of signal) were significantly different following the update, we adjusted for both in our analyses to determine whether it impacted our findings (described further in Results).
We used identical scan parameters pre- and post-update. Structural scans were collected using a T1 weighted sequence (MPRAGE) TR/TE = 1650/2.03ms, flip angle = 9°, FOV = 256 mm, slice thickness = 1 mm, sagittal plane, voxel size 1×1×1 mm. The functional scans used a T2-weighted gradient single-shot echo planar imaging sequence (TE = 25ms, TR = 2000 ms, flip angle = 90°, matrix 64×64) with an in-plane resolution of 3 × 3 mm (FOV = 220 mm) to acquire 33, 3 mm (interleaved) slices along the AC-PC plane. PACE was used as an in-scan prospective correction for movement (Thesen, Heid, Mueller, & Schad, 2000). Pillows, padding and headphones were used to restrict head motion. Researchers verbally checked in with children between anatomical and functional runs to ensure comfort and alertness.
Data Analysis
fMRI data processing
Processing of imaging data was carried out with BrainVoyager QX software (version 2.8, Brain Innovation, Maastricht, The Netherlands). Standard preprocessing steps were followed. First, spatial normalization was conducted to convert anatomical data to common stereotaxic space (i.e., Talairach) (Talairach & Tournoux, 1988) using the AC-PC landmark and fitting 6 parameters (anterior, posterior, inferior, superior, left, right) on each subject’s respective structural scan. Children’s anatomical data were converted to Talairach space so we could use coordinates reported in the more expansive adult literature to define our regions of interest. We justified use of common stereotaxic space based on reports that children (6 years and older) and adults have minimal differences in brain anatomy that are below the specificity of the scanner (Burgund et al., 2002), although we acknowledge the potential benefits of using study or child specific brain templates (Gaillard, Grandin, & Xu, 2001; Yoon, Fonov, Perusse, & Evans, 2009).
Functional data were preprocessed with 3D motion correction using 6 vectors (3 translations and 3 rotations) and temporal high-pass filtering using a GLM-Fourier basis set with 6 cycles per time course. Data were normalized by percent signal change to adjust for low-level physiological changes that occur during MRI scanning. The first two functional volumes were discarded for all participants. Functional runs with excess motion (cutoff: 3mm or 3° in any direction) were excluded from analyses. We did not smooth the data because of concerns about blurring anatomy in our ROI approach (Nieto-Castanon, Ghosh, Tourville, & Guenther, 2003). Pre-processed functional data were aligned and co-registered to anatomical data in Talairach space to create a volume time course file. Only subjects who had 1 or more successful functional run(s) were included in the analyses, resulting in 3 children excluded from final analyses. The final sample of n=47 ranged 3–6 successful runs, for an average of 5.1 successful
fMRI data were analyzed using a multi-subject random effects general linear model (GLM). Regressors included were conditions of PS (Large, Small) and ED (High, Low). Condition blocks were modeled by convolving the standard hemodynamic response function with a 30-second boxcar function. In a previous analysis published on the first 36 children from this cohort, we found no relationships between regions identified by a whole-brain analysis and the PSE. However, Berkman and Falk (Berkman & Falk, 2013) have advocated that the brain regions associated with behavior may be different from those that show significant effects based on tasks presented in the fMRI. To capture a broader range of regions implicated in appetitive behavior than what was identified by our whole-brain analyses, we instead chose an ROI approach to identify regions from the literature that have consistently be associated with food cue response. Regions were defined using Brain Voyager to draw a 5-mm sphere around Talairach coordinates previously reported in the food cue literature to be involved with reward/emotional processing (i.e., amygdala, ventral striatum, OFC, vmPFC) and inhibitory control/decision making (i.e., IFG/dorsolateral PFC, Table 3) (Brooks, Cedernaes, & Schiöth, 2013; Dimitropoulos et al., 2012; Hare, Malmaud, & Rangel, 2011; Schur et al., 2009; Stoeckel et al., 2008). We extracted mean beta values for the ROIs in response to the experimental conditions. Contrast values for each participant were then calculated by subtracting mean BOLD activation to one category of stimuli from the mean BOLD activation to another category (e.g., Large PS > Small PS; Large PS High ED > Small PS High ED; Large PS Low ED > Small PS Low ED). As an example, the Large PS > Small PS contrast value provides the difference in average BOLD activation to all Small PS images subtracted from the average activation to Large PS images (collapsed across ED) within the ROI. A child with a higher contrast value for Large PS > Small PS would therefore have comparatively greater BOLD signal in response to large relative to small portion cues compared with a child who had a lower contrast value for Large PS > Small PS.
Table 3.
Tailarach coordinates tested for association with the portion size effect
| Region of interest | Talairach Coordinates | |||
|---|---|---|---|---|
|
| ||||
| x | y | z | ||
| Amygdala d | R | 22 | −10 | −10 |
| L | −22 | −10 | −10 | |
| Striatum (ventral) d | R | 18 | 20 | −6 |
| R | −18 | 20 | −6 | |
| Orbitofrontal cortex b | R | 32 | 29 | −3 |
| L | −32 | 29 | −3 | |
| Orbitofrontal cortex d | R | 36 | 28 | −10 |
| L | −36 | 28 | −10 | |
| Ventromedial prefrontal cortex (vmPFC)c | R | 9 | 45 | 2 |
| L | −9 | 45 | 2 | |
| Ventromedial prefrontal cortex (vmPFC)c | R | 6 | 36 | −14 |
| L | −6 | 36 | −14 | |
| Inferior frontal gyrus (IFG) a | R | 50 | 4 | 16 |
| L | −50 | 4 | 16 | |
| Dorsolateral prefrontal cortex (dlPFC)a | R | 29 | 29 | 36 |
| L | −29 | 29 | 36 | |
| Caudatee | L | −14 | 8 | 22 |
| R | 14 | 8 | 22 | |
Brooks et al., (2013). PLoS One, 8(4)
Dimitropolous et al., (2012). Appetite, 58(1)
Hare et al., (2011). J Neurosci, 31(30)
Schur et al., (2009). Int J Obes, 33
Stoeckel et al., (2008). Neuroimage, 41(1)
In order to more clearly interpret the influence of including contrast values as covariates in mixed models, we reclassified them into two categories using a median split (e.g., high vmPFC response Large PS > Small PS versus low vmPFC response Large PS > Small PS). These categorical responses were input as covariates in mixed linear growth models to determine their effect on the shape of the PSE curve.
Mixed Effects Linear Growth Models
Previous studies have found that the PSE is curvilinear (Roe et al., 2016), with greater increases in intake occurring between smaller portion conditions (i.e., baseline and 150%) than between larger portion conditions (i.e., 175% and 200%). Because of the curvilinear nature of the PSE, it was not possible to model the response as a single value (i.e., the linear slope or the difference in intake between the largest and smallest conditions). Therefore, to test whether children’s brain response to food cues was associated with the PSE curve, we used mixed linear growth models. Mixed linear growth models allow examination of the shape of individual growth curves as a function of the amount of food served (Shek & Ma, 2011; Singer & Willett, 2003). By including contrast values obtained from BOLD fMRI as covariates, these models can be used to evaluate the impact of brain response to PS cues on the intercept (i.e., intake when PS condition is zero), linear slope (i.e., initial increases in intake from the baseline condition), and quadratic function (i.e., the curvilinear nature of the PS response curve observed at larger portion conditions). Estimates obtained from the statistical interaction term between BOLD response x weight of food served can be used to assess whether there is a relationship between food-cue related brain response and the PSE. Based on this information, we hypothesized that higher BOLD response in reward and drive related regions (i.e., amygdala, vmPFC, ventral striatum/caudate, OFC) to large relative to small PS cues would be associated with increased linear acceleration of the PSE curve, and because of this, would also be associated with a faster quadratic deceleration. In other words, we anticipated that heightened responses to large PS cues in appetitive-related regions would be associated with greater increases in intake from baseline followed by a curvilinear deceleration when amount served becomes too large. These responses would reflect children who find larger portions more salient, and therefore would exhibit a more robust increase in consumption when they are served larger portions in the laboratory. We also hypothesized that higher BOLD response in decision making and inhibitory control regions (i.e., IFG/dorsolateral PFC) to large relative to small PS cues would be associated with a reduced linear acceleration and slower quadratic deceleration of the PSE curve. This response pattern would be characteristic of children who show small increases in intake from baseline, but would require very large portions to reach peak consumption. In other words, this pattern would be indicative of attempts to moderate intake with increases in portion size.
Response to PS was defined as the trajectory of amount consumed (by weight) as a function of amount served (by weight) and modeled by a polynomial equation to take into account the decline in intake as portions become larger. A random coefficients model in SPSS (version 23.0 for Windows) was used to analyze the relationship between amount served (grams) and amount consumed (grams and kcal). Subjects were treated as a random factor. Amount served was modeled as both a linear and quadratic variable and was included in the models as both a fixed and a random factor. Additional fixed effects included BOLD signal contrast values computed from ROIs, input as categorical variables, and interactions between contrast values and amount served. Models were run with and without including children who finished greater than 95% of the total meal on any of the conditions (i.e., “plate cleaners”) to exclude the possibility that results were influenced by not offering children enough food. Related covariates, including sex, age, weight status (BMI z-score), average rated liking of the test-meal items, motion parameters for the fMRI (average motion in the x, y, and z planes concatenated across all runs), and fullness (computed as an average pre-meal fullness rating across the 4 meals), were entered independently into models as fixed factors, and removed if they were not significant. Signal-to-noise ratio (SNR) and TSNR from the fMRI scan were also included to determine if they influenced the relationship between brain response and the PSE. All interactions between covariates and PS condition were tested simultaneously and removed from the final model if not significant.
Pearson’s correlations between brain response to PS cues and BMI z-score
We also calculated the Pearson’s correlation coefficients between brain activation in the regions tested in response to the contrasts of interest (e.g., Large PS > Small PS; Large PS High ED > Small PS High ED; Large PS Low ED > Small PS Low ED) and child BMI z-score.
Data are reported as means ± standard deviations and/or standard errors and results were considered significant at a P < 0.05.
Results
Child characteristics
Demographic characteristics for the 47 children who completed all study measures are described in Table 1. Mean ± SD age was 8.9 ± 1.2 years and sex-specific BMI-for-age percentile was 52.7 ± 28.4. Seventeen percent (17%) of children were classified as overweight or obese, with a BMI-for-age ≥ 85th percentile. The majority of children, 93.6%, identified as Caucasian. Over 80% of families reported yearly earnings of $75,000 or greater, and 84.1% of parents reported having at least a Bachelor’s degree.
Effect of amount served on weight of food consumed
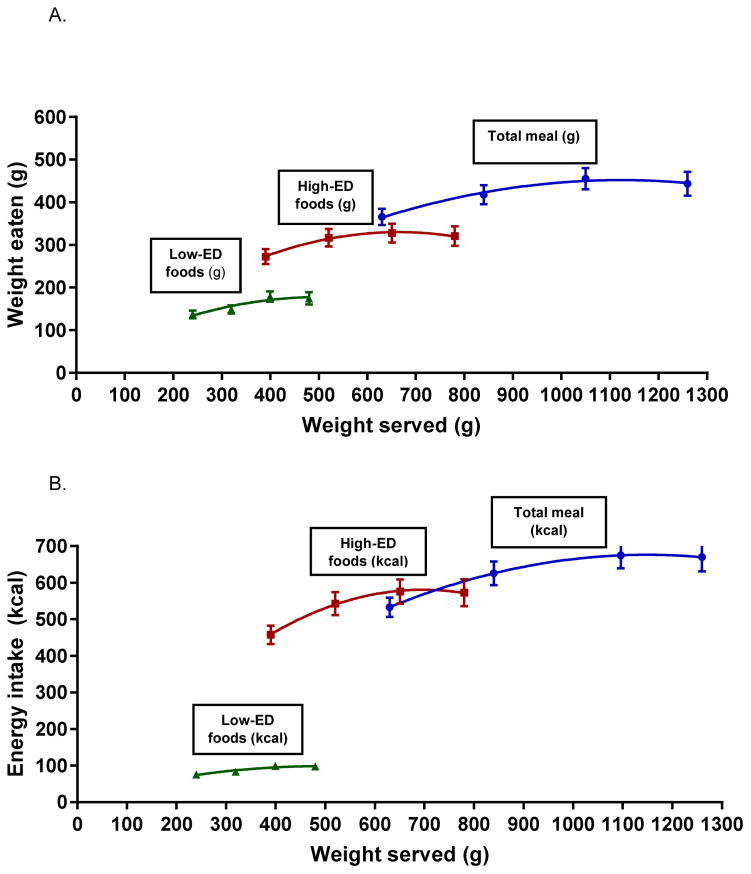
As shown by random coefficient analyses, amount served significantly influenced the weight of food consumed at the entire meal (F1,41=17.9; P < 0.0001) (Figure 1a). Across the entire sample, the relationship between weight served and weight consumed was characterized by a quadratic equation (Figure 1a). The Likelihood Ratio Test (P < 0.001) showed that a quadratic relationship fit the data better than a linear relationship. The mean curve had a positive linear coefficient (instantaneous slope) of 0.37 (P < 0.001) and a quadratic coefficient of −0.0004 (P < 0.005). According to the linear slope, as PS increased beyond the baseline, children ate an average of 37% of the additional food served, but this rate of intake was reduced by the quadratic coefficient at larger portion sizes. The pseudo-R2 value, assessed by a McFadden test, showed that amount served explained 26% of variability in weight of food consumed. These relationships were unchanged after removal of 1 child who was classified as a “plate cleaner”.
Figure 1.
a) Mean intakes by weight (± SEM) as a function of weight of food served (g) for the total meal and for high-ED and low-ED foods served to 47 children. Mean curves of food intake in response to increases in the weight of food served were modeled using a random coefficient analysis. Individual curves were modeled for each child. The weight of food served significantly influenced intake by weight of the total meal (F1,41=17.9; P < 0.0001), and of high-ED (F1,41=17.1; P < 0.0001) and low-ED (F1,41=8.9; P < 0.005) foods analyzed separately. There were no differences in the intake curves as a function of energy density. b) Mean intakes by kcal (± SEM) as a function of weight of food served (g) for the total meal and for high-ED and low-ED foods served to 47 children. Mean curves of energy intake in response to increases in the weight of food served were modeled using a random coefficient analysis. Individual curves were modeled for each child. The weight of food served significantly influenced energy intake of the total meal (F1,41=20.8; P < 0.0001), and of high-ED (F1,41=17.1; P < 0.0001) and low-ED (F1,41=8.9; P < 0.005) foods analyzed separately.
Amount served was also positively associated with intake of both high-ED (F1,41=17.1; P < 0.0001) and low-ED foods (F1,41=8.9; P < 0.005). The relationship between amount served and intake of high-ED foods was characterized by a quadratic equation (P < 0.001). The mean curve had a positive linear coefficient of 0.52 (P < 0.0001) and a quadratic coefficient of −0.0005 (P < 0.01). As amount served increased from baseline, children consumed 52% of the additional high-ED foods served, but this amount decreased over time as portions further increased. The relationship between amount served and intake of low-ED foods was characterized best by a linear equation (P < 0.01). The mean curve had a positive linear coefficient of 0.07 (P < 0.01) and a small, non-significant quadratic coefficient (−0.00004). Energy density of the foods did not significantly impact the curve parameters. Removal of plate cleaners did not influence these results.
Effect of food PS on energy intake
Weight of food served also significantly influenced energy intake at the total meal (F1,41=20.8; P < 0.0001), and the relationship was characterized by a quadratic equation (P < 0.005). The mean curve had a positive linear coefficient of 0.59 kcal/g (P < 0.0001) and a quadratic coefficient of −0.0006 kcal/g (P < 0.005). The pseudo-R2 value showed that amount served explained 26% of variability in energy consumed at the total meal. Weight of food served also influenced energy intake from high-ED foods (F1,41=17.1; P < 0.0001) and low-ED foods (F1,41=8.9; P < 0.01), analyzed separately. None of the above results were influenced by plate cleaners.
While the effect of weight of food served on both weight and energy consumed was significant, we observed individual variation in the PSE across children. The intraclass correlation showed that 71% of the total variation in the PSE was explained by between-subject variation. The purpose of the remaining analyses was to determine whether brain activation in a priori hypothesized regions was related to individual variations in the shape of the PSE curve across children.
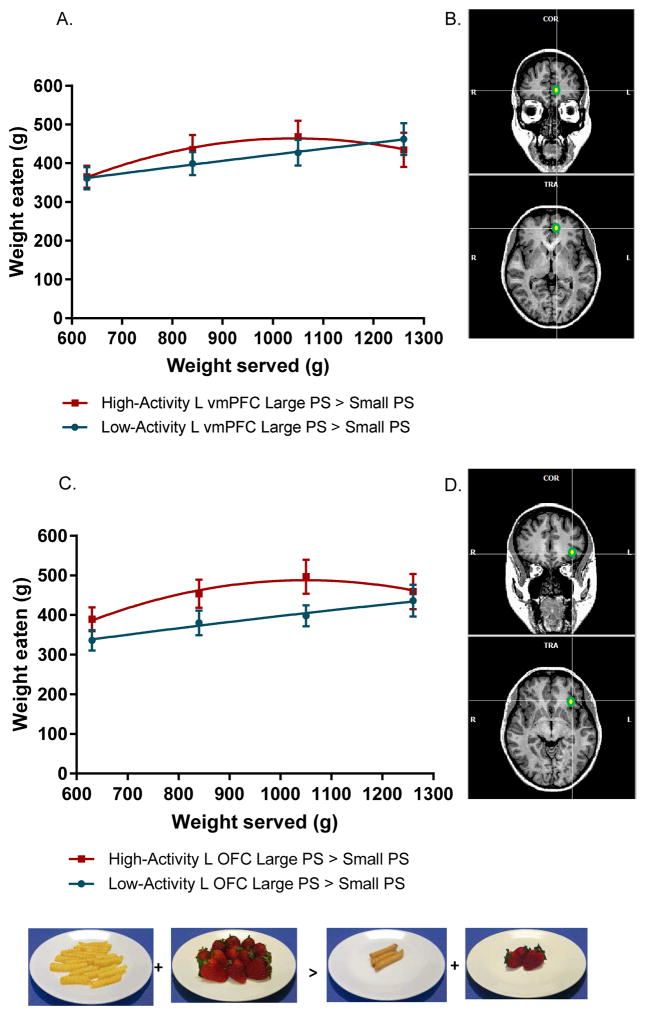
Associations between brain response to Large PS > Small PS and PSE response curve for the total meal
Activation in two brain regions associated with food salience was positively associated with children’s total meal intake in response to increasing portions. BOLD response in the left vmPFC (F1,41 = 5.70; P = 0.02) and the left OFC (F1,41 = 8.5; P = 0.004) in response to Large relative to Small PS food images interacted with weight of food served to influence the PSE curve (Figure 2). These relationships remained significant after inclusion of sex, age, BMI z-score, SNR, TSNR, fMRI motion, test-meal food liking, average pre-meal fullness level, and after removal of plate cleaners. For the vmPFC, high activation to Large relative to Small PS was positively associated with the linear slope (i.e., the increase in intake from baseline, 0.32; P ≤ 0.05) and a negative influence on the quadratic coefficient (i.e., the deceleration in intake as portion sizes become larger, −0.0006; P < 0.05) compared to children with low activation in this region. Children who had high activation in the vmPFC in response to larger PS cues increased intake from baseline by about 32% more than children who had low activation in this region. As a result of this initial increase, children who had high vmPFC activation also reached peak consumption at smaller portion sizes than children who had low vmPFC activation (as evidenced by the negative quadratic coefficient). A similar pattern of results was also seen for the OFC, as high activation to Large relative to Small PS was positively associated with the linear slope (0.14; P < 0.005) but negatively with the quadratic deceleration (−0.047; P < 0.01). None of the other regions tested (i.e., amygdala, striatum, IFG, dlPFC, and caudate) influenced the PSE curve for the total meal.
Figure 2.
a) Mean intakes by weight (± SEM) as a function of weight of food served (g) for the total meal for children who had high (red – square) or low (blue- circle) BOLD signal activation in the left vmPFC in response to Large PS > Small PS (collapsed across ED). High and low activation levels were categorized based on a median split. Activation in the vmPFC interacted with weight of food served to influence the trajectory of the PSE curve (F1,41 = 5.70; P = 0.02). Children who had high activation in the left vmPFC increased intake from baseline by 32% more than children who had low activation in the vmPFC in response to Large PS > Small PS. Overall, children with high vmPFC activation followed a curvilinear trajectory while children with low vmPFC activation followed a linear trajectory. b) Location of the ROI tested based on a 5-mm sphere drawn around the Talairach coordinates (x, y, z; −9, 45, 2) on a sample brain template created in BrainVoyager Brain Tutor (version 2.8, Brain Innovation, Maastricht, The Netherlands). Images are pictured from both the coronal (top) and transverse (bottom) views. c) Mean intakes by weight (± SEM) as a function of weight of food served (g) for the total meal for children who had high (red – square) or low (blue- circle) BOLD signal activation in the left OFC in response to Large PS > Small PS (collapsed across ED). High and low activation levels were categorized based on a median split. Activation in the OFC interacted with weight of food served to influence the trajectory of the PSE curve (F1,41 = 8.5; P = 0.004). Children who had high activation in the left OFC increased intake from baseline by 14% more than children who had low activation in the vmPFC in response to Large PS > Small PS. Overall, children with high OFC activation followed a curvilinear trajectory while children with low OFC activation followed a linear trajectory. d) Location of the ROI tested based on a 5-mm sphere drawn around the Talairach coordinates (x, y, z; −32, 29, −3) on a sample brain template created in BrainVoyager Brain Tutor (version 2.8, Brain Innovation, Maastricht, The Netherlands). Images are pictured from both the coronal (top) and transverse (bottom) views.
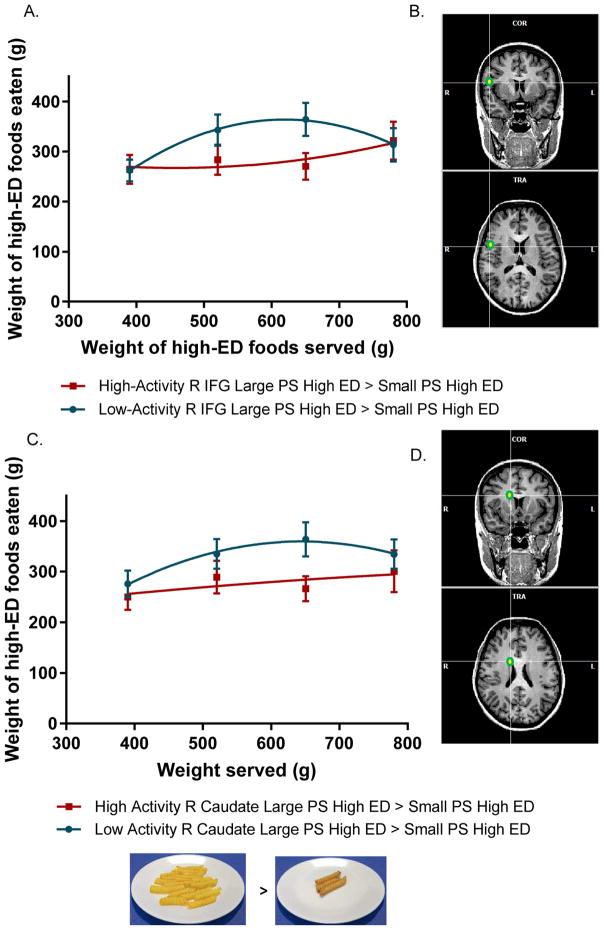
Associations between brain response to Large PS High ED > Small PS High ED and PSE response curve for high-ED foods
Activation in additional brain regions associated with cognitive control and reward processing was negatively associated with children’s intake of high-ED foods with increasing portions. In response to Large PS High ED > Small PS High ED, activation in the right IFG (F1,41 = 17.04; P < 0.0001), left OFC (F1,41 = 6.5; P = 0.02), and right caudate (F1,41 = 4.9; P = 0.03) interacted with amount of food served to influence the PSE curve for high-ED foods. Results for the IFG and caudate are displayed in Figure 3. The relationship in the IFG remained significant after correction for sex, age, fMRI motion, and BMI z-score, but was no longer significant after inclusion of pre-meal fullness and high-ED food liking (P = 0.06). Relationships in the OFC and caudate remained significant after inclusion of all covariates (i.e., sex, age, BMI z-score, fullness, SNR, TSNR, fMRI motion, and food liking). None of the above relationships were affected by dropping plate cleaners. For the IFG, high activation to Large PS High ED relative to Small PS High ED had a negative influence on the linear slope (−0.87; P < 0.0001) and was positively associated with quadratic coefficient (0.001; P < 0.0001). Children who had high activation in the IFG in response to large relative to small portions of high-ED foods consumed about 87% less from the baseline condition compared to children who had low activity in this region. A similar response pattern was seen in the caudate, as high activation to Large PS High ED > Small PS High ED had a negative influence on the linear slope (−0.57; P = 0.03) and a positive effect on the quadratic deceleration (0.0009; P = 0.03). Activation in the OFC in response to Large PS High ED relative to Small PS High ED was associated with the PSE curve, but in the opposite direction as the IFG and caudate. OFC activation was positively, but non-significantly associated with the linear slope (0.45; P = 0.08) and negatively associated with the quadratic coefficient (−0.001; P = 0.01). None of the other regions tested (i.e., amygdala, striatum, vmPFC, and dlPFC) influenced the PSE curve for high-ED foods.
Figure 3.
a) Mean intakes by weight (± SEM) as a function of weight of food served (g) for the total meal for children who had high (red – square) or low (blue- circle) BOLD signal activation in the right IFG in response to Large PS High ED > Small PS High ED. High and low activation levels were categorized based on a median split. Activation in the IFG interacted with weight of food served to influence the trajectory of the PSE curve (F1,41 = 17.04; P < 0.0001). Children who had low activation in the right IFG increased intake from baseline by 87% more than children who had high activation in the IFG in response to Large PS High ED > Small PS High ED. b) Location of the ROI tested based on a 5-mm sphere drawn around the Talairach coordinates (x, y, z; 50, 4, 16) on a sample brain template created in BrainVoyager Brain Tutor (version 2.8, Brain Innovation, Maastricht, The Netherlands). Images are pictured from both the coronal (top) and transverse (bottom) views. c) Mean intakes by weight (± SEM) as a function of weight of food served (g) for the total meal for children who had high (red – square) or low (blue- circle) BOLD signal activation in the right caudate in response to Large PS High ED > Small PS High ED. High and low activation levels were categorized based on a median split. Activation in the caudate interacted with weight of food served to influence the trajectory of the PSE curve (F1,41 = 4.9; P = 0.03). Children who had low activation in the right caudate increased intake from baseline by 57% more than children who had high activation in the caudate in response to Large PS High ED > Small PS High ED. Overall, children with high right caudate activation followed a linear trajectory while children with low right caudate activation followed a curvilinear trajectory. d) Location of the ROI tested based on a 5-mm sphere drawn around the Talairach coordinates (x, y, z; 14, 8, 22) on a sample brain template created in BrainVoyager Brain Tutor (version 2.8, Brain Innovation, Maastricht, The Netherlands). Images are pictured from both the coronal (top) and transverse (bottom) views.
Associations between brain response to Large PS Low ED > Small PS Low ED and PSE of low-ED foods
None of the regions tested (i.e., amygdala, striatum, vmPFC, dlPFC, OFC, IFG, or caudate) were associated with children’s PSE curve for low-ED foods served at the meal.
Associations between brain response to PS cues and child BMI z-score
The relationships between brain activation in response to PS cues and child BMI z-score are reported in Table 4 and Supplementary Figures 1–3. BMI z-score was negatively associated with brain activation in response to Large PS > Small PS in the right IFG (R = −0.29; P < 0.05) and left caudate (R = −0.36; P < 0.05). BMI z-score was positively associated with brain activation in response to Large PS High ED > Small PS High ED in the right (R = 0.43; P < 0.01) and left (R = 0.29; P < 0.05) ventral striatum, the left OFC (R = 0.38; P < 0.01) and the right (R = 0.31; P < 0.05) and left (R = 0.35; P < 0.05) vmPFC. BMI z-score was negatively associated with brain activation in response to Large PS Low ED > Small PS Low ED in the right (R = −0.48; P < 0.001) and left (R = −0.41; P < 0.01) ventral striatum, the left OFC (R = −0.31; P < 0.05), the left vmPFC (R = −0.39; P < 0.01), the left caudate (R = −0.31; P < 0.05), and the right (R = −0.36; P < 0.05) and left (R = −0.36; P < 0.05) dlPFC. None of the other ROIs tested were associated with child weight status for any of the contrasts [Table 4].
Table 4.
Correlations between brain response to portion size cues and child BMI z-score
| Contrast/Region | Tailarach Coordinates | Pearson’s R | P value | ||
|---|---|---|---|---|---|
| Large PS > Small PS | x | y | z | ||
| R Amygdala | 22 | −10 | −10 | 0.23 | 0.13 |
| L Amygdala | −22 | −10 | −10 | 0.22 | 0.13 |
| R Striatum (ventral) | 18 | 20 | −6 | −0.17 | 0.26 |
| L Striatum (ventral) | −18 | 20 | −6 | −0.16 | 0.28 |
| R Orbitofrontal cortex | 32 | 29 | −3 | 0.08 | 0.61 |
| L Orbitofrontal cortex | −32 | 29 | −3 | 0.22 | 0.15 |
| R Orbitofrontal cortex | 36 | 28 | −10 | −0.26 | 0.08 |
| L Orbitofrontal cortex | −36 | 28 | −10 | −0.17 | 0.25 |
| R vmPFC | 9 | 45 | 2 | 0.01 | 0.97 |
| L vmPFC | −9 | 45 | 2 | −0.04 | 0.81 |
| R vmPFC | 6 | 36 | 14 | 0.07 | 0.65 |
| L vmPFC | −6 | 36 | 14 | 0.12 | 0.44 |
| R IFG* | 50 | 4 | 16 | −0.29 | < 0.05 |
| L IFG | −50 | 4 | 16 | −0.10 | 0.55 |
| R dlPFC | 29 | 29 | 36 | −0.13 | 0.40 |
| L dlPFC | −29 | 29 | 36 | −0.15 | 0.32 |
| R Caudate | −14 | 8 | 22 | 0.08 | 0.58 |
| L Caudate* | 14 | 8 | 22 | −0.36 | < 0.05 |
| Large PS High ED > Small PS High ED | |||||
| R Amygdala | 22 | −10 | −10 | 0.18 | 0.21 |
| L Amygdala* | −22 | −10 | −10 | 0.29 | < 0.05 |
| R Striatum (ventral)** | 18 | 20 | −6 | 0.43 | < 0.01 |
| L Striatum (ventral)* | −18 | 20 | −6 | 0.29 | < 0.05 |
| R Orbitofrontal cortex | 32 | 29 | −3 | 0.21 | 0.16 |
| L Orbitofrontal cortex** | −32 | 29 | −3 | 0.38 | < 0.01 |
| R Orbitofrontal cortex | 36 | 28 | −10 | 0.003 | 0.99 |
| L Orbitofrontal cortex | −36 | 28 | −10 | 0.13 | 0.40 |
| R vmPFC* | 9 | 45 | 2 | 0.31 | < 0.05 |
| L vmPFC | −9 | 45 | 2 | 0.28 | 0.06 |
| R vmPFC | 6 | 36 | 14 | 0.16 | 0.29 |
| L vmPFC* | −6 | 36 | 14 | 0.35 | < 0.05 |
| R IFG | 50 | 4 | 16 | 0.001 | 0.99 |
| L IFG | −50 | 4 | 16 | 0.07 | 0.66 |
| R dlPFC | 29 | 29 | 36 | 0.24 | 0.10 |
| L dlPFC | −29 | 29 | 36 | 0.22 | 0.14 |
| R Caudate | −14 | 8 | 22 | −0.14 | 0.35 |
| L Caudate | 14 | 8 | 22 | −0.13 | 0.40 |
| Large PS Low ED > Small Low ED PS | |||||
| R Amygdala | 22 | −10 | −10 | 0.16 | 0.30 |
| L Amygdala | −22 | −10 | −10 | 0.13 | 0.40 |
| R Striatum (ventral)*** | 18 | 20 | −6 | −0.48 | < 0.005 |
| L Striatum (ventral)** | −18 | 20 | −6 | −0.41 | < 0.01 |
| R Orbitofrontal cortex | 32 | 29 | −3 | −0.14 | 0.36 |
| L Orbitofrontal cortex | −32 | 29 | −3 | −0.06 | 0.69 |
| R Orbitofrontal cortex | 36 | 28 | −10 | −0.25 | 0.09 |
| L Orbitofrontal cortex* | −36 | 28 | −10 | −0.30 | 0.04 |
| R vmPFC | 9 | 45 | 2 | −0.29 | 0.05 |
| L vmPFC** | −9 | 45 | 2 | −0.39 | < 0.01 |
| R vmPFC | 6 | 36 | 14 | −0.06 | 0.70 |
| L vmPFC | −6 | 36 | 14 | −0.21 | 0.16 |
| R IFG* | 50 | 4 | 16 | −0.23 | 0.11 |
| L IFG | −50 | 4 | 16 | −0.13 | 0.38 |
| R dlPFC* | 29 | 29 | 36 | −0.36 | < 0.05 |
| L dlPFC* | −29 | 29 | 36 | −0.36 | < 0.05 |
| R Caudate | −14 | 8 | 22 | 0.02 | 0.92 |
| L Caudate* | 14 | 8 | 22 | −0.31 | < 0.05 |
Discussion
The purpose of this study was to identify brain regions associated with children’s susceptibility to the effect of portion size at a multi-item meal. We hypothesized that activation in brain regions involved in inhibitory control and decision making (i.e., the IFG/dorsolateral PFC) would be negatively associated with PSE susceptibility, while activity in reward/salience regions (i.e., striatum/caudate, vmPFC, and OFC) would be positively associated with PSE susceptibility, particularly for high-ED foods. In confirmation of previous studies (Kling et al., 2016), PS influenced total meal intake, as well as intake of high- and low-ED foods analyzed separately. However, between-child differences explained over 70% of variance in the PSE. To determine whether brain responses to food cues contributed to some of this variance, we used mixed effects linear models. We identified two regions that were associated with the PSE for the total meal: the vmPFC (i.e., decision making and valuation) and the OFC (i.e., salience and associative learning). Secondary analyses identified brain regions implicated in inhibitory control (i.e., IFG) and reward/salience (i.e., caudate, OFC) that moderated the PSE specifically for high-ED foods. In general, the relationships with the PSE were supported by associations between brain response and child weight status in that regions we found to be positively associated with intake in response to PS were also associated with increased BMI z-score. These preliminary findings suggest that individual differences in the PSE among children are associated with brain responses to visual portion size cues in regions implicated in valuation, salience, inhibitory control, and reward.
For the total meal, greater activity in the vmPFC to larger PS cues was associated with increased susceptibility to the effect of portion size on intake. Children who had greater activity in this region to large relative to small PS cues increased intake to a greater extent from baseline, and as a result, they reached maximal consumption (i.e., peak of the curvilinear response) at smaller portion sizes than children who had lower activity. The vmPFC is involved in stimulus-reinforcement learning (Howard, Kahnt, & Gottfried, 2016) and assigning value judgements at the time of choice for food (Hare, Camerer, & Rangel, 2009; Hare et al., 2011) and other salient stimuli (Tom, Fox, Trepel, & Poldrack, 2007). Hare and colleagues (Hare et al., 2011) define value as the reward one expects to get from consuming a food. We speculate that children who engage the vmPFC to a greater extent when presented with larger portions might be conditioned to expect greater value from their consumption, and through repeated exposure, the brain may become hypersensitive to these visual portion size cues (i.e., incentive sensitization). The current findings preclude us, however, from determining whether increased activation in the vmPFC is driving food intake, or is simply a marker of increased susceptibility to overeating from larger portions. Alternatively, the functional connections between the vmPFC and dlPFC/IFG have been hypothesized to play a role in dietary self-control (Hare et al., 2009; Hare et al., 2011; Jasinska, Ramamoorthy, & Crew, 2011), therefore, connectivity studies are a useful next step to more comprehensively characterize the neurocircuitry associated with the PSE.
Similarly, BOLD response in the OFC to larger PS food cues was also positively associated with children’s intake in response to increasing portion sizes. The OFC is one of the most consistently identified brain regions associated with food cue processing (van Meer et al., 2015), and it is considered part of the appetitive network (Dagher, 2009). It is thought to be involved in integrating information from other classic reward regions (e.g., amygdala, caudate) to process the overall salience of a food (Padoa-Schioppa & Assad, 2008). It is also involved with perceptions of food pleasantness (Rolls & Grabenhorst, 2008) and cue-associated learning (Rolls & Grabenhorst, 2008; Rolls, Hornak, Wade, & McGrath, 1994). This region is adjacent to the vmPFC, and similarly, has also been reported to be involved with computations of food value at the time of choice (Plassmann, O’Doherty, & Rangel, 2007). These proposed functions may provide insight into factors that influence the effect of portion size on intake at a meal, and hence inform the neurobiological mechanisms of overeating in children. Previous studies have shown that adults (Brunstrom, 2014; Brunstrom & Rogers, 2009) and children (Hardman, McCrickerd, & Brunstrom, 2011) make decisions about how satiating a portion will be prior to a meal, and this correlates with actual meal consumption (Robinson, te Raa, & Hardman, 2015). These pre-meal decisions involve examining visual cues about the food and making determinations, based on prior experience, in regards to how satiating one expects a portion to be. Considering this evidence along with the present findings, the vmPFC and OFC may be associated with the PSE through their involvement in assigning expected satiation values to meals prior to consumption.
Secondary analyses were performed to identify potential differences in brain response that moderated the PSE for high- versus low-ED foods. For high-ED foods, we predicted that greater response to portion size would be positively associated with BOLD response in regions implicated in reward, but negatively associated with response in regions implicated in inhibitory control. Our findings in the IFG supported this prediction, as greater activation in this region to high-ED foods shown in larger portions was associated with reduced susceptibility to increases in portion size for similar types of foods at the meal. The IFG is located within the lateral prefrontal cortex, a region implicated in inhibitory control and attention (Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010). The exact location we tested is at the posterior edge of the IFG, near the operculum and insular cortex (Frank, Kullmann, & Veit, 2013). This region, referred to as the “ingestive cortex” responds to multiple food attributes, including taste (Small et al., 2003), texture (Ivan E. de Araujo & Rolls, 2004), visual characteristics (Frank et al., 2010; Porubská, Veit, Preissl, Fritsche, & Birbaumer, 2006) and stimulus intensity (Grabenhorst & Rolls, 2008). Activity in this region is modulated by appetitive state, as it receives primary afferent information from the gut, which may facilitate its involvement in interoception of bodily sensations, including hunger and fullness (Craig, 2002). Our findings in this region were partly explained by pre-meal fullness level, suggesting a possible role integrating food-cue information with appetitive state to influence meal-size. Collectively, these findings suggest that increased food-cue activity in this region of the IFG near the insular cortex/frontal operculum may be associated with reduced susceptibility to the PSE.
We also found a similar pattern of response in the caudate (i.e., higher food cue activation was associated with reduced susceptibility to the PSE at smaller portion conditions). This was not expected, given the proposed role of the caudate in reward processing (O’Doherty, Deichmann, Critchley, & Dolan, 2002). These findings support the reward-deficit model whereby overconsumption occurs to compensate for a “sluggish” or impaired reward response (Blum et al., 1996; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008; Stice, Yokum, Blum, & Bohon, 2010). Alternatively, as speculated by Kroemer and Small (Kroemer & Small, 2016), these findings could reflect impairment in value-based learning among individuals at risk for overeating.
Although it was not a primary objective of this study, we also examined the relationships between brain activation in response to PS cues and child weight status. In general, these data supported the relationships observed with test-meal intake. For example, activation in the IFG and caudate in response to images of large relative to small portions was negatively associated with child weight status which underscores our finding that PS-cue reactivity in these regions was associated with reduced susceptibility to the PSE. Importantly, though, the observed differences in the PSE were independent of child weight status, as the models were adjusted for this variable and child BMI z-score was not significantly associated with children’s intake at the meals. Therefore, children’s brain responses to PS cues were independently associated with both intake at the test-meals and weight status. When we analyzed brain activation in response to high- and low-ED food cues separately, we found contrasting relationships to child weight status. Activation in regions associated with reward and salience (e.g., striatum, OFC, vmPFC) to large relative to small portions of high-ED foods was positively associated with child weight status. Alternatively, activation in these same regions to large relative to small portions of low-ED foods was negatively associated with weight status. These findings support others who have shown that adults and children with obesity have greater food cue related activation in some reward-related regions, like the striatum and OFC (Bruce et al., 2010; Dimitropoulos et al., 2012; Stice et al., 2008; Stoeckel et al., 2008), but they extend the literature by suggesting that greater activation in similar reward-processing regions to larger portions of healthier (low-ED) foods may be associated with leanness.
We previously published a paper using a whole-brain approach (English et al., 2017) that found a different region of the IFG located in Brodmann’s Area 47 (Brodmann, 2006), near the lateral OFC, was associated with lower activation to large relative to small PS cues. Post-hoc tests demonstrated this result was driven primarily by reduced activation to large portions of low-ED foods, but we did not find activation in this region to be associated with appetitive traits or the PSE for the total meal (English et al., 2017). One likely explanation is that the region of the IFG previously identified by whole-brain analysis has different functions than the region tested in the present study. The IFG region tested in the present ROI approach was selected based on coordinates reported in a meta-analysis of adults, which suggests reliable correlations to anticipatory responses to food cues across a variety of studies and paradigms (Brooks et al., 2013). That location of the IFG corresponds to Brodmann’s area 44 and is near the insula, a region involved in taste (de Araujo & Simon, 2009) and interoception (Craig, 2002). Although we identified both sub-regions of the IFG using different analytical approaches, we speculate that engagement of these areas is likely occurring in concert along with connections to additional regions within the ingestive networks of the brain to ultimately influence the observed behavioral responses. For that reason, studying the connections between these regions is a necessary future step to determine their respective roles in processing information about food portion size and influencing subsequent eating behaviors. The comparison of the current findings with our previously reported results (English et al., 2017) at a minimum reinforces the need for considering multiple possible functions for brain regions when interpreting results and relying on carefully designed replication studies to strengthen conclusions.
This is the first study to report an association between brain response to PS cues and children’s susceptibility to overeating from increasing portions. Few neuroimaging studies have been conducted with children under the age of 8 years, so these data fill a critical gap in knowledge about the mechanisms of overeating in this age group. Aside from the results in the caudate, the findings generally support the notion that increased food-cue induced response in brain regions implicated in reward and motivation positively associated with intake in response to increasing portions, while activation in regions implicated in cognitive control and interoception negatively associated with intake in response to increasing portions. Once these findings are replicated, they may help to identify potential neural and behavioral targets for future interventions to help children moderate overeating in an environment where large portions are ubiquitous.
Other strengths include development of a carefully designed, ecologically relevant set of fMRI stimuli that varied by both ED and PS, and objective measurement of food intake in response to PS across four meal conditions. We also observed high scanner success rates for this age group, likely due to a carefully designed mock training protocol. Despite these strengths, several limitations should be noted. We did not measure brain response to the foods simultaneously with consumption of the test-meal, and although technically challenging, this design may provide greater insight about the neurobiological drivers of the PSE. Also, the portion sizes for the foods shown to children in the fMRI were not identical to those served at the meal because we wanted to maximize differences in BOLD response between conditions. In addition, we did not adjust our findings for multiple testing because the effect sizes observed at the test-meal were not large enough to withstand these corrections, beyond the results in the IFG and OFC. Larger, more generalizable studies are needed to confirm these findings. The ROI approach we took to analyze the data has the benefit of being hypothesis-driven; however, we could have overlooked additional brain areas with involvement in the PSE. Additionally, we used common stereotaxic space (Talairach) to compare our findings with other relevant examples in the field (Bruce et al., 2010), but the anatomical precision afforded by spatial normalization procedures is limited (Carp, 2012). Replication studies are needed to make stronger conclusions about the brain regions associated with the PSE in children. Finally, we measured the PSE in response to a test-meal of common, age-appropriate foods. We do not know whether children’s intake in response to this meal generalizes to other foods or eating occasions.
In conclusion, activation to food PS cues in regions implicated in value judgements, salience and reward positively associated with children’s intake in response to increasing portions, while activation in regions implicated in control negatively associated with intake in response to increasing portions. These findings provide initial support for the characterization of neurobiological phenotypes at risk for overeating in response to large portions.
Supplementary Material
Table 2.
Foods and amounts offered to children in four test-meal conditions
| Food | Energy Density | Amount Served (g) | |||
|---|---|---|---|---|---|
| kcal/g | 100% condition | 133% condition | 167% condition | 200% condition | |
| High Energy Dense | |||||
| Macaroni and cheesea | 1.5 | 300 | 400 | 500 | 600 |
| Garlic breadb | 3.6 | 50 | 67 | 83 | 100 |
| Angel food cakec | 2.5 | 40 | 53.3 | 67.7 | 80 |
| Low Energy Dense | |||||
| Broccoli with butter | 0.4 | 90 | 120 | 150 | 180 |
| Cherry tomatoes | 0.2 | 50 | 66.7 | 83.3 | 100 |
| Red grapes | 0.7 | 100 | 133 | 166 | 200 |
| Total meal | 1.5 | 630 | 840 | 1050 | 1260 |
Stouffer’s macaroni and cheese, Nestlé USA, Solon, OH, USA.
Pepperidge Farm, Inc., Norwalk, CT, USA.
Sara Lee, Downer’s Grove, IL, USA.
Acknowledgments
The authors thank the children and families for participation in this study. We also thank Liane Roe, MPH, RDN for her assistance with statistical analyses. This study was supported by the Penn State Social Sciences Research Institute, National Center of Research Resources and the National Center for Advancing Translational Sciences, NIH, Grant UL1TR000127. Doctoral training for co-authors LKE and SNF was supported by the USDA Childhood Obesity Prevention Training Grant #2011670013011. Imaging was conducted at the Penn State Social, Life, & Engineering Sciences Imaging Center 3T MRI Facility.
Footnotes
Clinical trials registration: The study is registered on www.ClinicalTrials.gov NCT02759523.
Conflict of Interest: The authors declare no conflict of interest
- LKE, SNF, SJW, JOF, BJR and KLK designed research (project conception, development of overall research plan, and study oversight);
- LKE, SNF, KA, and ML conducted research (hands-on conduct of the experiments, data collection and pre-processing)
- KLK, SNF, LKE, and MB analyzed data and performed statistical analysis
- KLK wrote paper with input from co-authors, including LKE, SNF, and BJR
- KLK has primary responsibility for final content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. doi: http://dx.doi.org/10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond Brain Mapping: Using Neural Measures to Predict Real-World Outcomes. Current directions in psychological science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;39:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Brodmann’s Localisation in the Cerebral Cortex: The Principles of Comparative Localisation in the Cerebral Cortex Based on Cytoarchitectonics. New York, NY: Springer; 2006. [Google Scholar]
- Brooks SJ, Cedernaes J, Schiöth HB. Increased Prefrontal and Parahippocampal Activation with Reduced Dorsolateral Prefrontal and Insular Cortex Activation to Food Images in Obesity: A Meta-Analysis of fMRI Studies. PloS one. 2013;8(4):1–9. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International journal of obesity (2005) 2010;34(10):1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Lepping RJ, Bruce JM, Cherry JB, Martin LE, Davis AM, Brooks WM, Savage CR. Brain Responses to Food Logos in Obese and Healthy Weight Children. J Pediatr. 164(4):759–64. doi: 10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Brunstrom JM. Mind over platter: pre-meal planning and the control of meal size in humans. International Journal of Obesity. 2014;38:S9–S12. doi: 10.1038/ijo.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom JM, Rogers PJ. How Many Calories Are on Our Plate? Expected Fullness, Not Liking, Determines Meal-size Selection. Obesity. 2009;17(10):1884–1890. doi: 10.1038/oby.2009.201. [DOI] [PubMed] [Google Scholar]
- Brunstrom JM, Rogers PJ, Pothos EM, Calitri R, Tapper K. Estimating everyday portion size using a ‘method of constant stimuli’: In a student sample, portion size is predicted by gender, dietary behaviour, and hunger, but not BMI. Appetite. 2008;51(2):296–301. doi: 10.1016/j.appet.2008.03.005. doi: http://dx.doi.org/10.1016/j.appet.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The Feasibility of a Common Stereotactic Space for Children and Adults in fMRI Studies of Development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. doi: http://dx.doi.org/10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Carnell S, Benson L, Pantazatos SP, Hirsch J, Geliebter A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity. 2014;22(11):2370–2378. doi: 10.1002/oby.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. On the Plurality of (Methodological) Worlds: Estimating the Analytic Flexibility of fMRI Experiments. Frontiers in Neuroscience. 2012;6(149) doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Opinion: How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes. 2009;33(S2):S30–S33. doi: 10.1038/ijo.2009.69. [DOI] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Lotze M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity. 2010;34(1):94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET. Representation in the Human Brain of Food Texture and Oral Fat. The Journal of Neuroscience. 2004;24(12):3086–3093. doi: 10.1523/jneurosci.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes. 33(S2):S34–S43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58(1):303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English L, Lasschuijt M, Keller KL. Mechanisms of the portion size effect. What is known and where do we go from here? Appetite. 2015;88:39–49. doi: 10.1016/j.appet.2014.11.004. doi: http://dx.doi.org/10.1016/j.appet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- English LK, Fearnbach SN, Lasschuijt M, Schlegel A, Anderson K, Harris S, Wilson SJ, Fisher JO, Savage JS, Rolls BJ, Keller KL. Brain regions implicated in inhibitory control and appetite regulation are activated in response to food portion size and energy density in children. Int J Obes. 2016 doi: 10.1038/ijo.2016.126. [DOI] [PubMed] [Google Scholar]
- English LK, Fearnbach SN, Wilson SJ, Fisher JO, Savage JS, Rolls BJ, Keller KL. Food portion size and energy density evoke different patterns of brain activation in children. Am J Clin Nutr. 2017;105:10. doi: 10.3945/ajcn.116.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JO. Effects of Age on Children’s Intake of Large and Self-selected Food Portions. Obesity. 2007;15(2):403–412. doi: 10.1038/oby.2007.549. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Arreola A, Birch LL, Rolls BJ. Portion size effects on daily energy intake in low-income Hispanic and African American children and their mothers. The American journal of clinical nutrition. 2007;86(6):1709–1716. doi: 10.1093/ajcn/86.5.1709. [DOI] [PubMed] [Google Scholar]
- Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children’s intake at a meal. The American journal of clinical nutrition. 2007;86(1):174–179. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Frontiers in Human Neuroscience. 2013;7(499) doi: 10.3389/fnhum.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullman S, Veit R, Carnova C, Hegner YL, Preissl H. Processing of food pictures: influence of hunger, gender, and calorie content. Brain Research. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B. Developmental Aspects of Pediatric fMRI: Considerations for Image Acquisition, Analysis, and Interpretation. NeuroImage. 2001;13(2):239–249. doi: 10.1006/nimg.2000.0681. doi: http://dx.doi.org/10.1006/nimg.2000.0681. [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Bell J, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD. Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience. 2009;30(8):1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. European Journal of Neuroscience. 2008;27(3):723–729. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. doi: http://dx.doi.org/10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CA, McCrickerd K, Brunstrom JM. Children’s familiarity with snack foods changes expectations about fullness. The American journal of clinical nutrition. 2011;94(5):1196–1201. doi: 10.3945/ajcn.111.016873. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing Attention on the Health Aspects of Foods Changes Value Signals in vmPFC and Improves Dietary Choice. The Journal of Neuroscience. 2011;31(30):11077–11087. doi: 10.1523/jneurosci.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JD, Kahnt T, Gottfried JA. Converging prefrontal pathways support associative and perceptual features of conditioned stimuli. Nat Commun. 2016:7. doi: 10.1038/ncomms11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Ramamoorthy A, Crew CM. Toward a Neurobiological Model of Cue-Induced Self-Control in Decision Making: Relevance to Addiction and Obesity. The Journal of Neuroscience. 2011;31(45):16139–16141. doi: 10.1523/jneurosci.4477-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Assur SA, Torres M, Lofink HE, Thornton JC, Faith MS, Kissileff HR. Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite. 2006;47(2):233–243. doi: 10.1016/j.appet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Young AD, Femia LA, Bogorodzki P. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage (Orlando, Fla) 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Developmental Psychobiology. 2005;47(4):377–397. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- Kling SMR, Roe LS, Keller KL, Rolls BJ. Double trouble: Portion size and energy density combine to increase preschool children’s lunch intake. Physiology & Behavior. 2016;162:18–26. doi: 10.1016/j.physbeh.2016.02.019. doi: http://dx.doi.org/10.1016/j.physbeh.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral TV, Rolls BJ. Energy density and portion size: their independent and combined effects on energy intake. Physiol Behav. 2004;82:131–138. doi: 10.1016/j.physbeh.2004.04.063. [DOI] [PubMed] [Google Scholar]
- Kral TVE, Kabay AC, Roe LS, Rolls BJ. Effects of doubling the portion size of fruit and vegetable side dishes on children’s intake at a meal. Obesity. 2010;18(3):521–527. doi: 10.1038/oby.2009.243. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Small DM. Fuel not fun: reintepreting attenuated brain responses to reward in obesity. Physiology & Behavior. 2016;162:37–45. doi: 10.1016/j.physbeh.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Yun-Hee K, Nobre AC, Marsel M. Hunger Selectively Modulates Corticolimbic Activation to Food Stimuli in Humans. Behavioral Neuroscience [PsycARTICLES] 2001;115(2):493–493. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Leahy KE, Birch LL, Rolls BJ. Reducing the Energy Density of an Entrée Decreases Children’s Energy Intake at Lunch. Journal of the American Dietetic Association. 2008;108(1):41–48. doi: 10.1016/j.jada.2007.10.015. doi: http://dx.doi.org/10.1016/j.jada.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, … Savage CR. Neural Mechanisms Associated With Food Motivation in Obese and Healthy Weight Adults. Obesity. 2009;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Mathias KC, Rolls BJ, Birch LL, Kral TVE, Hanna EL, Davey A, Firsher JO. Serving larger portions of fruits and vegetables together at dinner promotes intake of both foods among young children. J Acad Nutr Dietet. 2012;112(2):266–270. doi: 10.1016/j.jada.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooreville M, Davey A, Orloski A, Hannah EL, Mathias KC, Birch LL, Kral TV, Zakeri IF, Fisher JO. Individual differences in susceptibility to large portion sizes among obese and normal-weight children. Obesity. 2015;23(4):808–814. doi: 10.1002/oby.21014. [DOI] [PubMed] [Google Scholar]
- Nielsen SJ, Popkin BA. Patterns and trends in food portion sizes. JAMA. 2003;289:450–453. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. NeuroImage. 2003;19(4):1303–1316. doi: 10.1016/s1053-8119(03)00188-5. doi: http://dx.doi.org/10.1016/S1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nature Neuroscience. 2008;11(1):95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal Cortex Encodes Willingness to Pay in Everyday Economic Transactions. The Journal of Neuroscience. 2007;27(37):9984–9988. doi: 10.1523/jneurosci.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubská K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: An fMRI study. NeuroImage. 2006;32(3):1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. doi: http://dx.doi.org/10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. THE ROLE OF EARLY LEFT-BRAIN INJURY IN DETERMINING LATERALIZATION OF CEREBRAL SPEECH FUNCTIONS. Annals of the New York Academy of Sciences. 1977;299(1):355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Robinson E, te Raa W, Hardman CA. Portion size and intended consumption. Evidence for a pre-consumption portion size effect in males? Appetite. 2015;91:83–89. doi: 10.1016/j.appet.2015.04.009. doi: http://dx.doi.org/10.1016/j.appet.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Roe LS, Kling SMR, Rolls BJ. What is eaten when all of the foods at a meal are served in large portions? Appetite. 2016;99:1–9. doi: 10.1016/j.appet.2016.01.001. doi: http://dx.doi.org/10.1016/j.appet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Morris EL, Roe LS. Portion size of food affects energy intake in normal-weight and overweight men and women. Am J Clin Nutr. 2002;76(6):1207–1213. doi: 10.1093/ajcn/76.6.1207. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity (Silver Spring, Md) 2007;15(6):1535–1543. doi: 10.1038/oby.2007.182. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Functions of the anterior insula in taste, autonomic, and related functions. Brain and Cognition. doi: 10.1016/j.bandc.2015.07.002. doi: http://dx.doi.org/10.1016/j.bandc.2015.07.002. [DOI] [PubMed]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. doi: http://dx.doi.org/10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57(12):1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes. 2009;33(6):653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek DTL, Ma CMS. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures, and illustrations. The Scientific World Journal. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: modeling change and event occurrence. New York, NY: Oxford University Press, Inc; 2003. [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Smiciklas-Wright H, Mitchell D, Mickle S, Cook A, Goldman J. Foods commonly eaten in the United States: Quantities consumed per eating occasion and in a day, 1994–96. 2002. Retrieved from Beltsville, MD. [Google Scholar]
- Spill MK, Birch LL, Roe LS, Rolls BJ. Eating vegetables first: the use of portion size to increase vegetable intake in preschool children. Am J Clin Nutr. 2010;91(5):1237–1243. doi: 10.3945/ajcn.2009.29139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity. Journal of Abnormal Psychology. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::AID-MRM17>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The Neural Basis of Loss Aversion in Decision-Making under Risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture, A. R. S. N. D. L. USDA Nutrient Database for Standard Reference. 2015 Release 28. Retrieved from http://www.ars.usda.gov/ba/bhnrc/ndl.
- van Meer F, van der Laan LN, Adan RAH, Viergever MA, Smeets PAM. What you see is what you eat: An ALE meta-analysis of the neural correlates of food viewing in children and adolescents. NeuroImage. 2015;104:35–43. doi: 10.1016/j.neuroimage.2014.09.069. doi: http://dx.doi.org/10.1016/j.neuroimage.2014.09.069. [DOI] [PubMed] [Google Scholar]
- Yoon U, Fonov VS, Perusse D, Evans AC. The effect of template choice on morphometric analysis of pediatric brain data. NeuroImage. 2009;45(3):769–777. doi: 10.1016/j.neuroimage.2008.12.046. doi: http://dx.doi.org/10.1016/j.neuroimage.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Young LR, Nestle M. Expanding portion sizes in the US marketplace: implications for nutrition counseling. J Am Diet Assoc. 2003;103(2):231–234. doi: 10.1053/jada.2003.50027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.