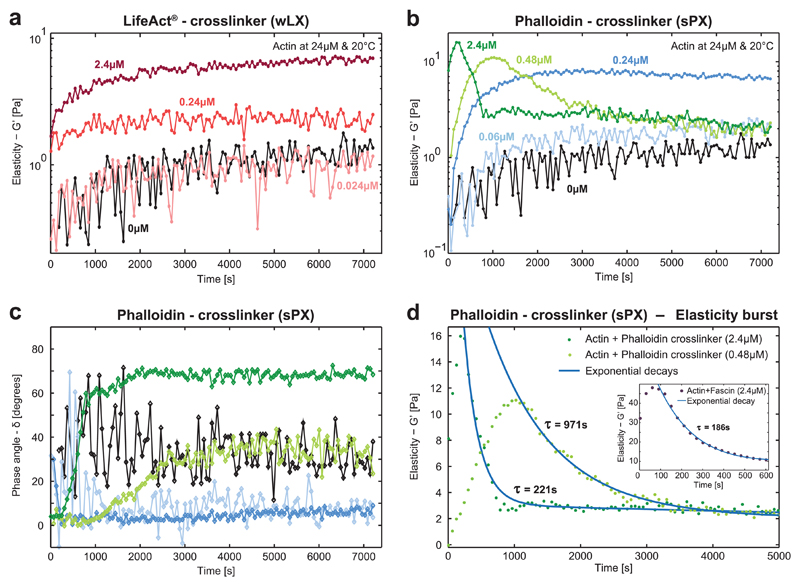
Figure 2. Time evolution of the elasticity during actin polymerization in the presence of synthetic actin crosslinkers.
a) Mechanical properties of actin networks (24 × 10−6 m) enriched with different concentrations (R = 0, 0.001, 0.01, 0.1) of the wLX were determined via dynamic shear rheology. Measurements always started with monomeric actin and the monotonically increasing G′ of pure actin (black) illustrates the formation of filaments and their arrangements into entangled networks. Upon addition of the crosslinker, the elasticity increased in a concentration-dependent manner similar to the natural crosslinker α-actinin (corresponding crosslinker concentrations are given in different shades of red). b) Strong phalloidin crosslinkers induced entirely different mechanical fingerprints comparable to findings reported for the strong natural crosslinker fascin.[14,22] Networks are initially monotonically stiffened similar to wLX, but at much lower concentrations (R = 0, 0.0025, 0.01, 0.02, 0.1). When reaching a concentration threshold, the time evolution of G′ became nonmonotonic. First, the elasticity drastically increased until reaching a peak, which was subsequently followed by an exponential decay—a time evolution that is referred to as the “elasticity burst.”[14] c) The corresponding phase angles illustrate that low crosslinker concentrations (blue curves) induced a predominantly elastic response. High crosslinker densities initially induced a predominant elastic response (green curves), but in the time evolution the systems became increasingly viscous. d) The higher sPX concentration (dark green) induced the elasticity burst earlier with a higher magnitude of G′ with a fast subsequent relaxation indicated by the shorter decay time. Inset: Similar characteristic fingerprints have been observed in actin networks with a high concentration of the natural crosslinker fascin.[14]