Figure 3. Convergence of PR nuclear and extranuclear signaling pathways to regulate biological responses.
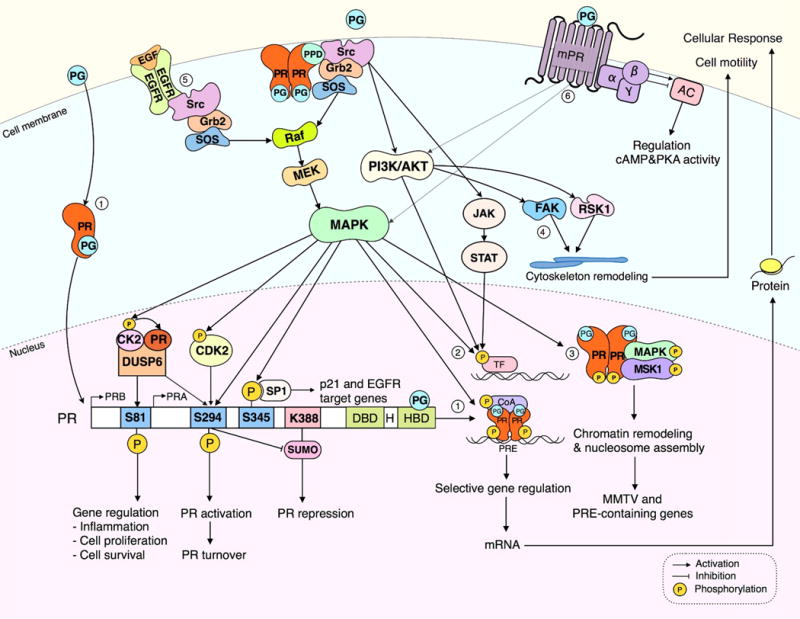
In the classic nuclear signaling pathway (1) progesterone activates progesterone receptor (PR) by binding and inducing conformational changes of the receptor causing in turn nuclear translocation, dimerization and binding to progesterone response elements (PRE) in the promoters or enhancer regions of PR target genes. Progestin treatment rapidly activates extranuclear signaling of a subpopulation of PR localized in the membrane or cytoplasm to transiently associate with c-Src through interaction with the PR polyproline domain (PPD) leading to activation of mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K/Akt), or Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathways. Activated MAPK further regulates PR transcriptional activity through phosphorylation of PR, co-activators (1) or other transcription factors (2). Rapid progestin activation of MAPK can directly phosphorylate PR or co-activators (1) or promote PR phosphorylations through CK2 and CDK2 (see text for details). Alternatively, activation of various cytoplasmic signaling pathways may phosphorylate and increase transcriptional activity of other transcription factors, independent of PREs (2). Rapid extranuclear activation of ER/PR complexes by progestins activates MAPK leading to a formation of phospho-PR/MAPK/Msk1 (Mitogen and stress-activated protein kinase 1), chromatin remodeling and enhanced MMTV and PRE-containing gene transcription (3). PR extranuclear activation of Src/PI3K/Akt may stimulate focal adhesion kinase (FAK) or ribosomal S6 kinase 1 (RSK1) triggering actin cytoskeleton remodeling and promotion of cell motility (4). Alternatively, PR extranuclear signaling may cross-communicate with growth factor signaling such as epidermal growth factor (EGF) leading to activation of MAPK and downstream events (5). Membrane localized progestin receptor (mPR) unrelated to the classical PR mediates progestin extranuclear signaling through GPCR-like membrane proteins via modulation of adenylate cyclase (AC), cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). mPR has been reported to activate MAPK and PI3K/Akt signaling pathways. More work is needed to define the physiological significance of extranuclear mPR activation of these several cytoplasmic signaling pathways in BC cells.
