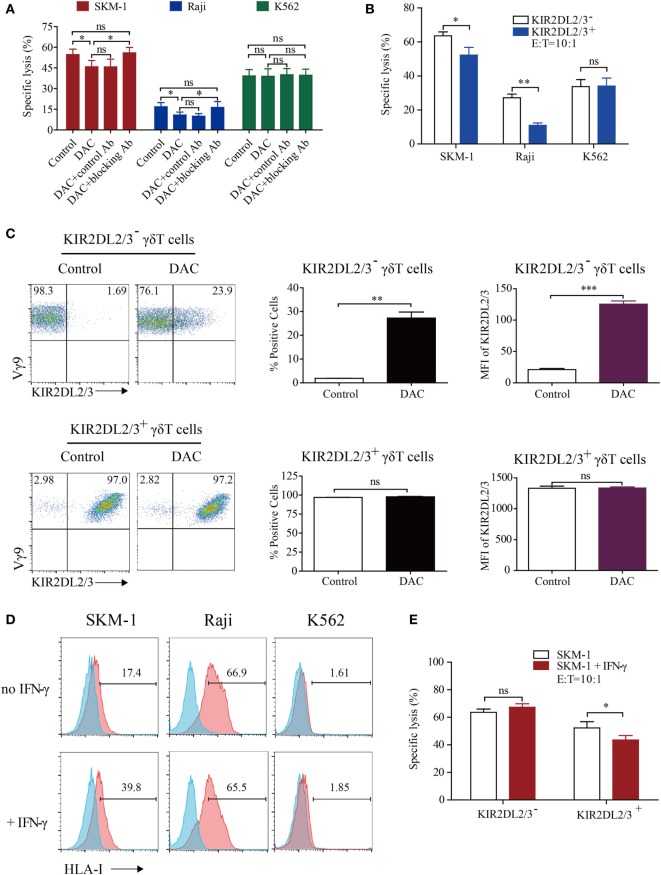
Figure 3.
Decitabine induced polarization of KIR2DL2/3-negative gamma delta (γδ) T cells to KIR2DL2/3-positive cells, which are less cytotoxic to tumor cells expressing HLA-I molecules than KIR2DL2/3-negative γδ T cells. (A) The decrease in γδ T cell killing was inhibited by the KIR2DL2/3-blocking antibody (Ab). The 0.5 µM DAC-treated γδ T effector cells were first treated with or without Abs to KIR2DL2/3 for 30 min and then incubated with target cells at 37°C for 4 h to test the cytotoxicity (*p < 0.05; ns, not significant; n = 3). (B) The cytotoxicity of KIR2DL2/3-positive and -negative γδ T cells to tumor cells. KIR2DL2/3-positive and -negative γδ T cells were incubated with Raji, SKM-1, and K562 cells at 37°C for 4 h to test the cytotoxicity (n = 3; *p < 0.05; **p < 0.01; ns, not significant). (C) Effect of DAC on KIR2DL2/3 expression on KIR2DL2/3-positive and -negative γδ T cells. KIR2DL2/3-positive and -negative γδ T cells were sorted from cultured γδ T cells and treated with 0.5 µM DAC for 48 h. FACS dot plots showing the changes of KIR2DL2/3 on KIR2DL2/3-positive and -negative γδ T cells after treatment with DAC. Graph showing the percentage and mean fluorescence intensity (MFI) of KIR2DL2/3 on KIR2DL2/3-positive and -negative γδ T cells after treatment with DAC (**p < 0.01; ***p < 0.001; ns, not significant; n = 3). (D) HLA-I molecule expression on tumor cells and interferon (IFN)-γ-treated tumor cells. Tumor cells were first treated with 200 IU/mL IFN-γ for 48 h, and then harvested and stained with mouse anti-human HLA-I molecular Ab. (E) The cytotoxicity of KIR2DL2/3-positive and -negative γδ T cells to SKM-1 cells and IFN-γ-treated SKM-1 cells (*p < 0.05; ns, not significant; n = 3).