Figure 1.
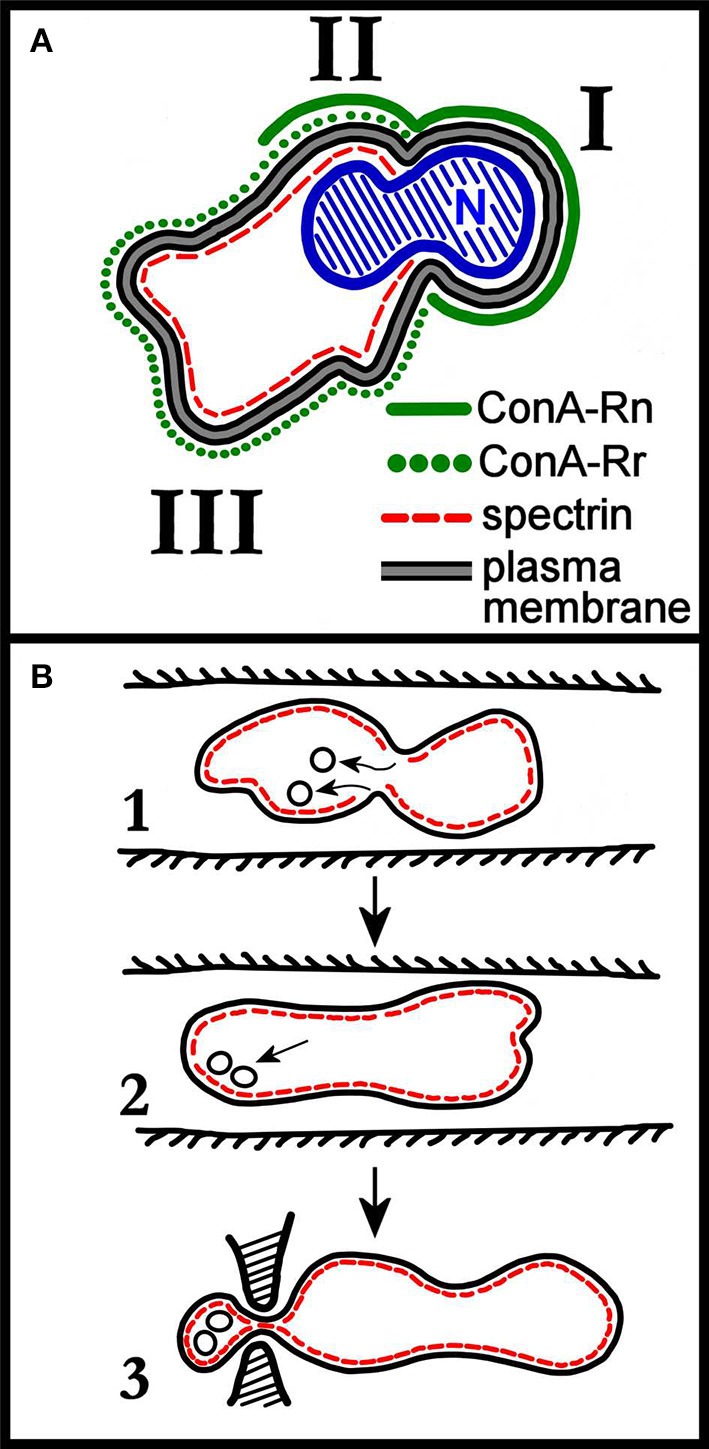
(A) Schematic distribution of membrane proteins in the enucleating mouse erythroblast. Roman numerals I, II, and III correspond to the domains identified by the authors of the work (Geiduschek and Singer, 1979). Domain III corresponds to the incipient reticulocyte. The nucleus (N), not completely extruded, shows the characteristic constriction which separates domain I from domain II. As discussed in the text of the cited work, two classes of ConA receptors (ConA is a lectin that binds to band3, so ConA receptors are band 3 molecules) are assumed to be present in the erythroblast membrane, with ConA-Rn (but not ConA-Rr) present in domain I; both ConA-Rn and ConA-Rr in the membrane of domain II; and ConA-Rr (but not ConA-Rn) in domain III. Most strikingly, spectrin is confined to domains II and III. Re-drawn from Geiduschek and Singer (1979). (B) Rudimentary early depiction of a model for the remodeling of the cell membrane during retic maturation to RBC in vivo. Panel 1 depicts the invaginations of spectrin-free domains of the retic membrane occurring in the circulation, and their subsequent endocytosis. In panel 2, the endocytosed vesicles are pictured as associating with the membrane either to fuse with it or to be exocytosed. In panel 3, the exocytosis of vesicles in a larger, spectrin-containing body is pictured as occurring by mediation of the spleen. Re-drawn from Zweig et al. (1981).
