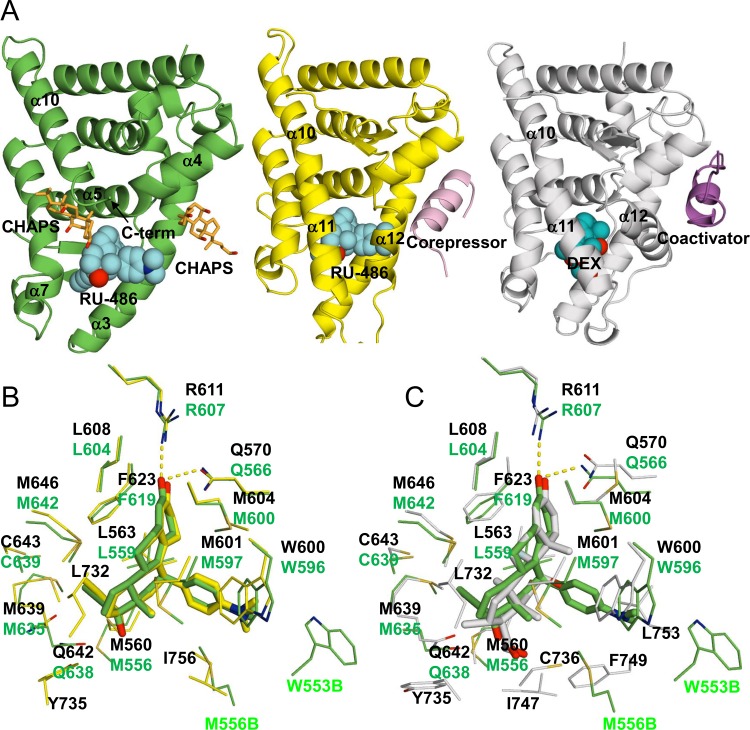
FIG 6.
Comparison of GRα and β structures. (A) nmrGRβ/RU-486 (left), hGRα/RU-486 (PDB ID 3H52, middle), and hGRα/DEX (PDB ID 1M2Z, right) complex structures. Two CHAPS molecules bound to nmrGRβ are shown in orange. Corepressor and coactivator peptides bound to hGRα are shown in pink (middle) and purple (right), respectively. RU-486 (cyan) and DEX (cobalt) are shown as spheres. (B) Superposition of the ligand binding pockets of the hGRα/RU-486 (yellow) and nmrGRβ/RU-486 (green) complex structures. Selected residues interacting with the ligands are shown. Residue numbers from hGRα/RU-486 and nmrGRβ/RU-486 complex are shown in black and green, respectively. (C) Superposition of the ligand binding pockets of hGRα/DEX (white) and nmrGRβ/RU-486 (green) complex structures. M556B and W553B are from molecule B of the nmrGRβ asymmetric unit.