Figure 1.
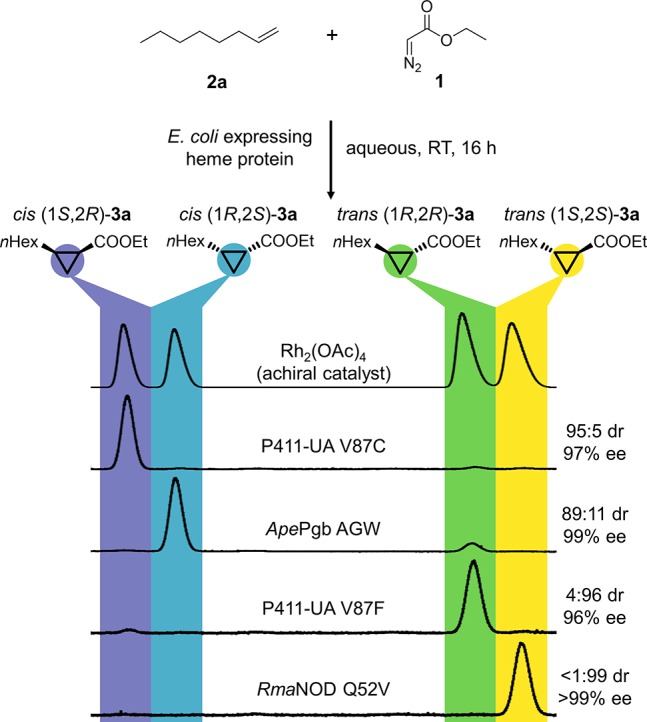
Stereoselective enzymatic cyclopropanation of the aliphatic alkene 2a and 1 to obtain each of four stereoisomers of cyclopropane product 3a with diastereoselectivies from 89:11 to <99:1 dr and enantioselectivies from 96% to >99% ee. Reaction conditions: whole E. coli cells in M9-N buffer, 25 mM glucose, 10 mM 2a, direct addition of 20 mM 1 under anaerobic conditions, 5% ethanol cosolvent. The diastereoselectivity ratio (dr) is given as cis:trans, and the enantiomeric excess (ee) is given for the major diastereomer. Catalysts used: rhodium acetate dimer (Rh2(OAc)4) to form the racemic authentic standard, two variants of the engineered, serine-ligated cytochrome P450BM3 (P411-UA-V87C and P411-UA-V87F), Aeropyrum pernix protoglobin W59A Y60G F145W (ApePgb AGW), and Rhodothermus marinus nitric oxide dioxygenase Q52V (RmaNOD Q52V). Protein sequences are available in the Supporting Information. Abbreviations used: RT, room temperature; nHex, n-hexyl.
