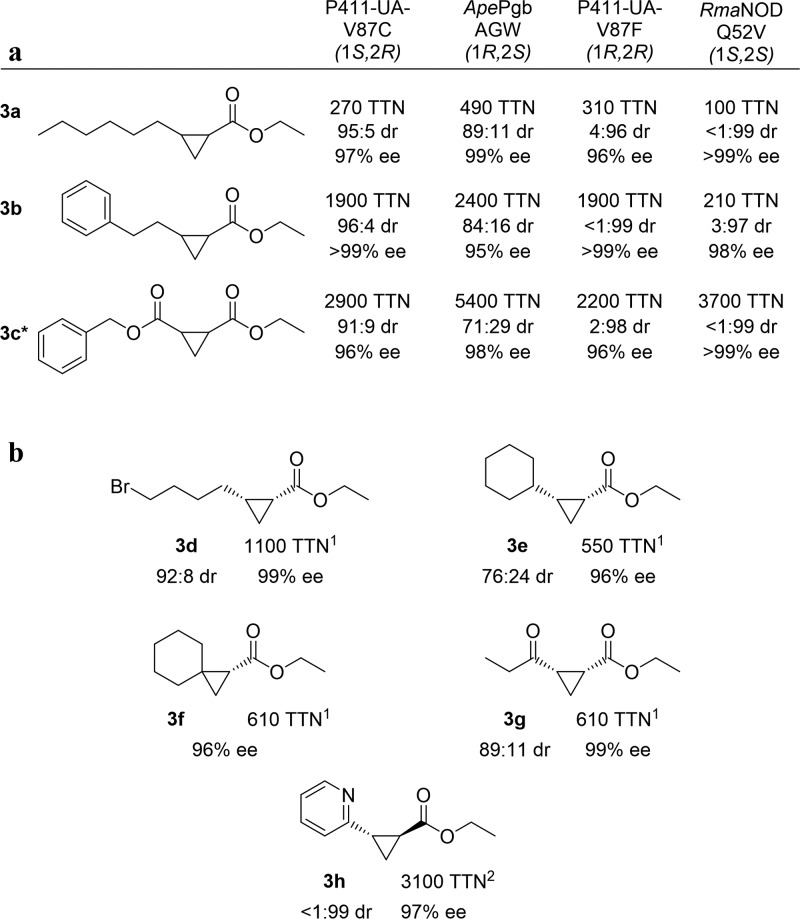
Figure 2.
Cyclopropanation substrate scope. (a) Activity and selectivity of each protein variant against 3a, 3b, and 3c. (b) Activity and selectivity against a variety of alkenes. The diastereoselectivity ratio (dr) is given as cis:trans, and the enantiomeric excess (ee) is given for the major diastereomer. Enzyme variant used is denoted by superscripts: 1, ApePgb AGW; 2, RmaNOD Q52V. General reaction conditions: whole E. coli cells (OD600 = 5 (ApePgb AGW, RmaNOD Q52V), OD600 = 20 (P411-UA-V87C, P411-UA-V87F)) in M9-N buffer, 25 mM glucose, 10 mM alkene, direct addition of 20 mM 1 under anaerobic conditions, 5% ethanol cosolvent. Modified reaction conditions: 3g OD600 = 20; 3e OD600 = 10. RmaNOD Q52V 3a, OD600 = 10. Analytical yields for these reactions are given in Supplemental Table 8. The absolute configurations of products 3b and 3c are assigned by analogy to the 3a products (see the Supporting Information section “Determination of absolute configurations of the cyclopropane products” for details). Chiral separation conditions reported in the Supporting Information. *The benzyl ester of 3c has IUPAC naming priority, and therefore the chiral carbon numbering is reversed for these compounds.