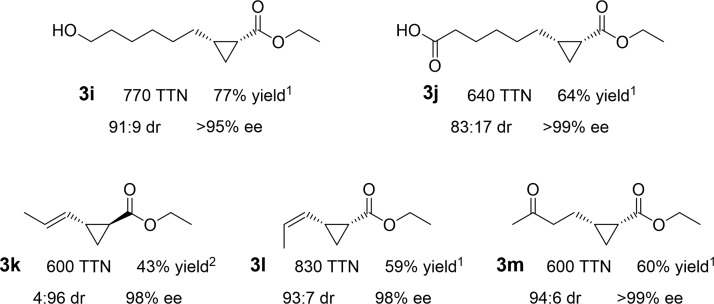
Figure 3.
Selective, preparative-scale cyclopropane syntheses from various aliphatic alkenes and dienes. Preparative-scale reactions against substrates with free alcohol (7-octen-1-ol, 2i), free carboxylic acid (7- octen-1-oic acid, 2j), the two geometric isomers of 1,3-pentadiene (2k, 2l), and ketone (5-hexen-2-one, 2m). The diastereoselectivity ratio (dr) is given as cis:trans, and the enantiomeric excess (ee) is given for the major diastereomer. The yields are reported for isolated products. Enzyme variant used is denoted by superscripts: 1, ApePgb AGW; 2, RmaNOD Q52V. Reaction and chiral separation conditions are available in the Supporting Information.