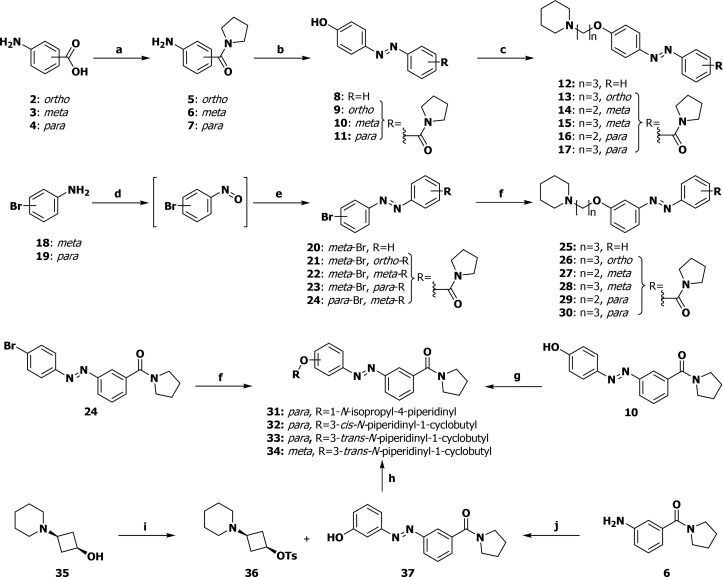
Scheme 1. General Synthetic Scheme for Photoswitchable H3R Antagonists.
Reagents and conditions: (a) pyrrolidine, EDCI·HCl, DIPEA, HOBt·H2O, DMF, rt, 16 h, 57–95%; (b) (I) NaNO2, 1 M aq HCl, 0 °C, 5 min; (II) phenol, aq NaOH, rt, 30 min; (III) 1 M aq HCl, aq satd NH4Cl, rt, 10 min, 30–81%; (c) NaI, K2CO3, Pip-(CH2)n-Cl·HCl, DMF, 130 °C, 16 h, 31–76%; (d) Oxone, H2O/DCM 4:1, rt, 3 h; (e) R-Ar-NH2, AcOH/DCM 1:1, rt, 16 h, 30–76% (over two steps); (f) R-OH, RockPhos, [PdCl(C3H5)]2, Cs2CO3, PhMe, 90 °C, 23 h, 12–47%; (g) R-OH, DEAD, PPh3, THF, 0 °C→ rt, 18 h, 33–73%; (h) 1-methylimidazole, 4-MePhSO2Cl, DCM, rt, 48 h, 43%; (i) (I) Oxone, H2O/DCM 4:1, rt, 6 h; (II) 3-OTBMDS-aniline, AcOH, rt, 16 h; (III) TBAF, THF, 0 °C, 10 min, 13%; (j) (I) NaH, DMF, rt, 30 min; (II) 36, 90 °C, 16 h, 59%; Pip = 1-piperidino.