Fig. 1.
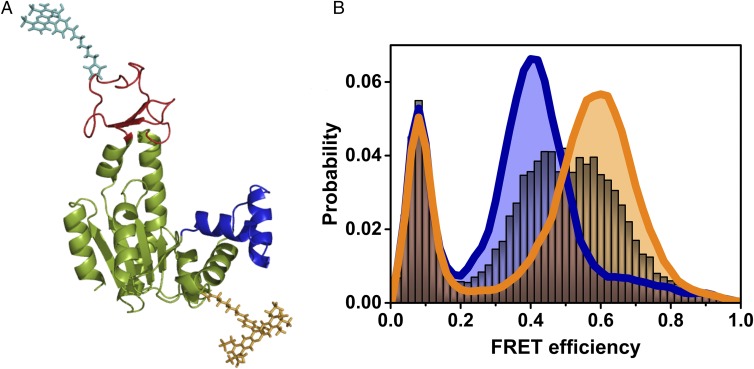
Single-molecule studies of the conformational dynamics of AK. (A) Structure of the open conformation of AK, with the LID domain in red and the NMPbind domain in blue (based on PDB ID code 4AKE). Donor (cyan) and acceptor (dark yellow) dyes attached to the CORE and LID domains, respectively, are depicted. (B) FRET efficiency histograms of AK in the absence of substrate (royal blue) and in the presence of saturating substrate concentrations (1 mM ATP, 1 mM AMP, and 160 µM ADP, orange), suggesting mostly open and closed conformations, respectively. In the presence of 2.5 μM ATP (with 1 mM AMP and 8.4 μM of ADP), a single broad peak is observed (gray), indicating fast exchange between open and closed conformations. The population with FRET efficiency < 0.2 is due to molecules without an active acceptor.