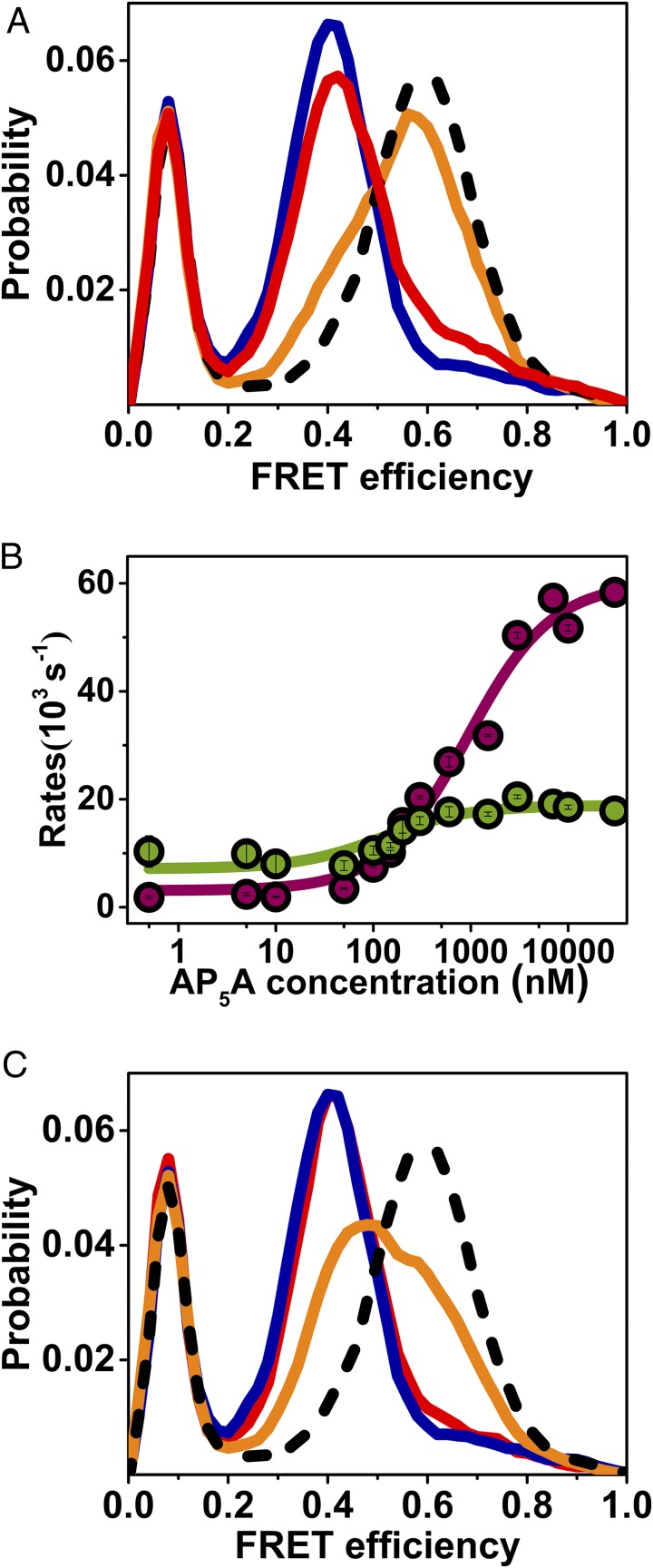
Fig. 3.
smFRET experiments shed light on mechanistic aspects of domain closure. (A) Substrate binding to both domains is required for domain closure. FRET efficiency histograms of AK in the absence of substrate (royal blue), with 50 μM ATP (red), with 50 μM ATP and 1 mM AMP (orange), and with 50 μM ATP, 1 mM AMP, and a suitable concentration of ADP to maintain equilibrium (dashed black line). (B) The two-substrate mimicking inhibitor, AP5A, leads to a concentration dependence of the closing and opening rates (cherry and green, respectively) similar to ATP. (C) The nonhydrolyzable ATP analog, AMP-PNP, does not fully shift the equilibrium to the closed conformation even at a high concentration. At 50 μM AMP-PNP and 1 mM AMP (red), the FRET efficiency histogram is similar to the one without any substrate (royal blue), while at 1 mM AMP-PNP and 1 mM AMP (orange), the histogram has shifted only halfway to the position obtained with 1 mM ATP and 1 mM AMP and a suitable amount of ADP (dashed black line).