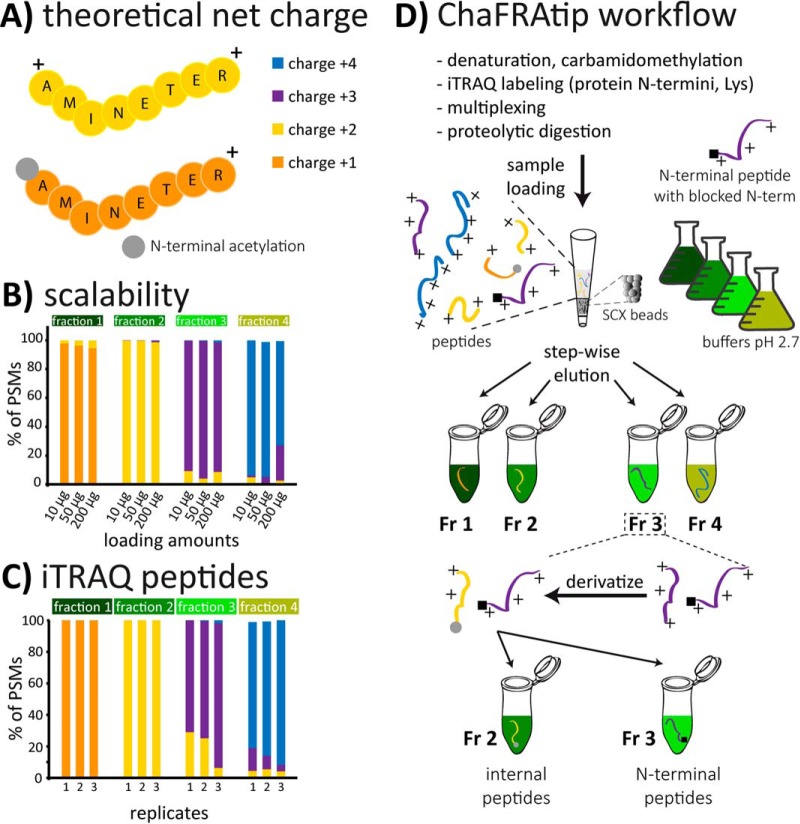
Fig. 1.
(A) The theoretical net charge (TNC) of a fully tryptic peptide at pH 2.7 is typically +2. Presence of His residues or missed cleavages leads to increased TNC, N-terminal acetylation to reduced TNC. (B) Tip-based fractionation of peptides according to their TNC can be scaled to the amount of cell lysate without compromising separation power and (C) also works for iTRAQ-labeled peptides. (D) For ChaFRAtip, free protein N termini and lysines are labeled with iTRAQ/TMT on the protein level; samples are pooled and digested with trypsin. Peptides are fractionated on-tip according to their TNC. Each collected fraction is acetylated, thus reducing the TNC of internal peptides by 1. In a second tip-based fractionation, internal peptides with reduced TNC are removed and N-terminal peptides are collected for subsequent LC-MS analysis.