Fig. 1.
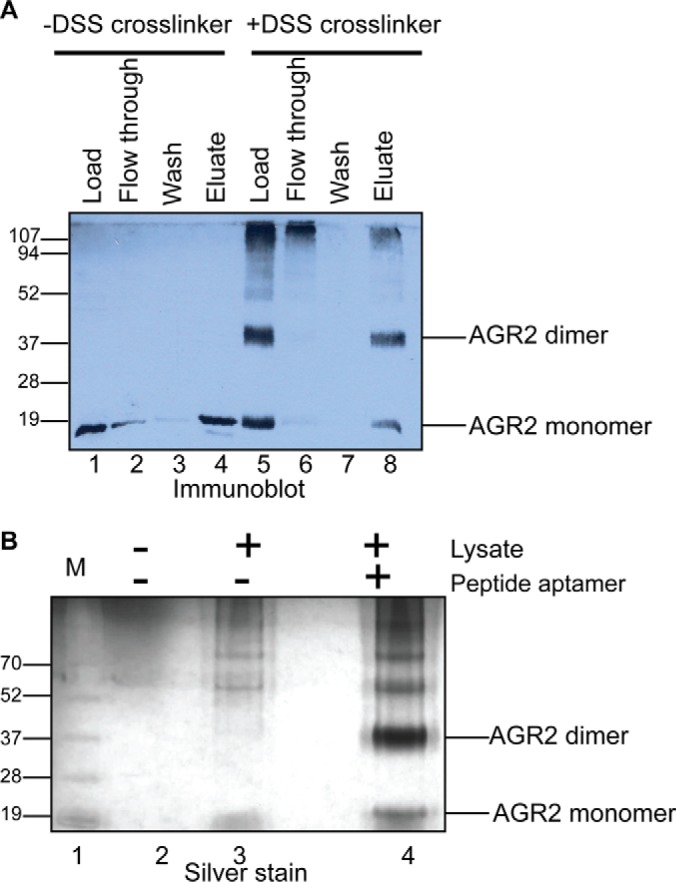
The specificity of an AGR2-binding peptide aptamer for use in the affinity purification of in vivo cross-linked AGR2 protein from crude lysates. Affinity purification of AGR2 from crude cell lysates. MCF7 cells grown in media with 10% FCS were incubated with DMSO (lanes 1–4) or with a fixed concentration of the cell-membrane permeable cross-linker DSS (lanes 5–8) for 1 h at 37 °C. Cells were harvested, lysed using a Tris-HCl (pH 8.0) buffer containing 1% Nonidet P-40, lysates were incubated with an optimized biotinylated peptide aptamer (named A4) linked to streptavidin beads, which can be used to affinity purify the AGR2 protein in crude cell lysates (24). The input lysates (Load, lanes 1 and 5), flow-through fractions (lanes 2 and 6), washes (lanes 3 and 7), and eluates (lanes 4 and 8) were separated by electrophoresis. The protein in the polyacrylamide gel was (A) immunoblotted to determine whether intermediary species of cross-linked AGR2 could be affinity purified and (B) stained with silver to measure total protein captured. In part B, streptavidin beads were incubated with buffer only (lane 1), lysates (lane 2), and lysates with preconjugated peptide-A4-beads (lane 3). The 18 kDa and 36 kDa cross-linked and affinity purified silver-stained proteins (arrows, lane 3 versus lane 2) were excised using trypsin and AGR2 protein was confirmed present by MALDI-TOF mass spectrometry (data not shown; dimeric excised band; only human AGR2 peptides were detected, Score = 161; queries matched = 4; emPAI = 0.86).
