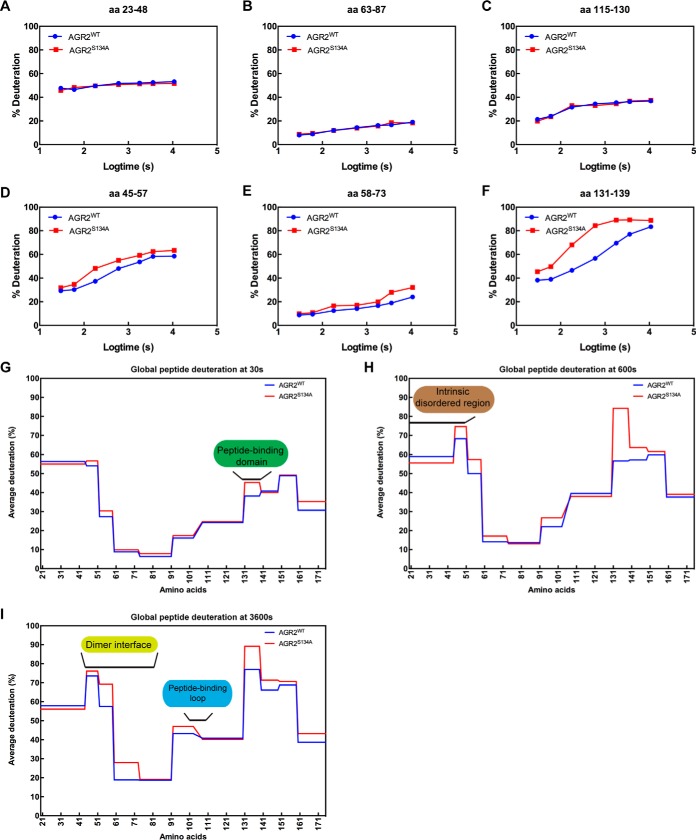
Fig. 12.
Measuring conformational changes in the gain-of-function mutant AGR2S134A using hydrogen-deuterium mass spectrometry. Wt-AGR2 or AGR2S134A was deuterated over a 7-point time course from 30 s to 10,800 s followed by acidification, pepsinization, and separation of fragments using mass spectrometry (supplemental Fig. S3) A–C, Representative peptic ions of AGR2 protein that do not exhibit significant changes in deuteration between wt and mutant AGR2S134A D–F, Representative peptic ions of AGR2 protein that do exhibit significant changes in deuteration between wt and mutant AGR2S134A. The data are plotted as % of deuteron exchange as a function of time (log10 in seconds; from 30, 60, 180, 600, 1800, 3600, and 10800). The deuterium exchange rates of individual peptides are summarized using the HDX exchange plots for (G) 30 s (H) 600 s, and (I) 3,600 s time course. In (G), we highlight that the most noticeable difference is enhanced deuteration at the “peptide binding domain”, including amino acids 131–135. However, at elevated times of deuteration (H–I), there is apparent exposure of the dimerization domain and the peptide-binding loop to solvent, suggestive of global conformational changes induced by the S134A mutation.