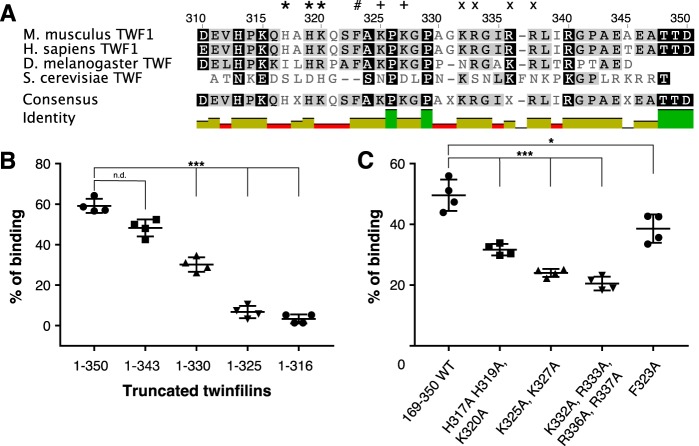
Figure 3.
Clusters of positively charged residues in the tail of twinfilin facilitate its lipid interactions. A, a sequence alignment of tail regions from mouse and human twinfilin-1 as well as fruit fly and budding yeast twinfilins. Clusters of positively charged residues mutated to alanines are highlighted with *, +, and ×. The conserved phenylalanine mutated for biochemical experiments is highlighted with #. B, a vesicle cosedimentation assay preformed on full-length twinfilin-1 and C-terminally truncated proteins. C, vesicle cosedimentation assays carried out on wild-type twinfilin-1 and mutant proteins. The final protein and lipid concentrations were 2 and 500 μm, respectively, and the lipid composition was POPC:POPS:POPE:PI(4,5)P2 50:20:20:10. Results are shown as individual data points and as the mean of four experiments. Error bars represent standard deviations. Statistical significance was calculated with Student's t test. ***, p < 0.001; *, p < 0.05. n.d., not detectable.