Figure 7.
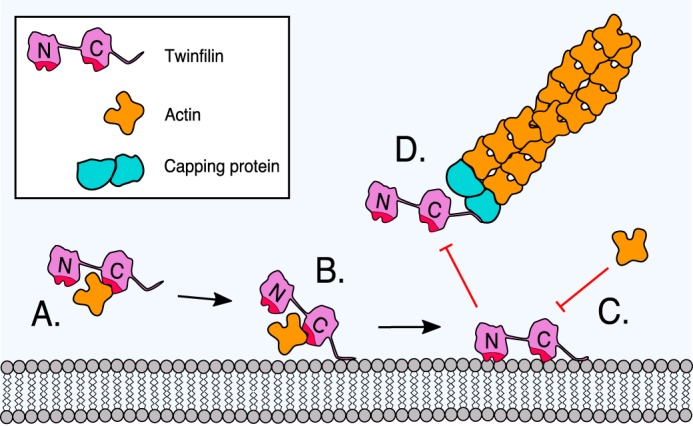
A working model for the inhibition of twinfilin-1 by a phosphoinositide-rich membrane. A, twinfilin-1 interacts, through its C-terminal ADF-H domain, with an ADP-actin monomer and prevents its association with filament ends. B, the C-terminal tail of twinfilin-1 can anchor the protein onto a phosphoinositide-rich membrane through electrostatic interactions between the positively charged residues of the protein and negatively charged lipid headgroups. C, membrane-tethering (driven by the C-terminal tail) places the actin-binding ADF-H domains of twinfilin in contact with the membrane. Because the ADF-H domains associate with phosphoinositide through an interface that overlaps with the actin-binding sites, this leads to inhibition of the actin-binding activities of twinfilin-1. D, because the binding sites for phosphoinositides and capping protein overlap in the tail of twinfilin-1, phosphoinositide binding is also expected to inhibit the interaction of twinfilin with capping protein.
