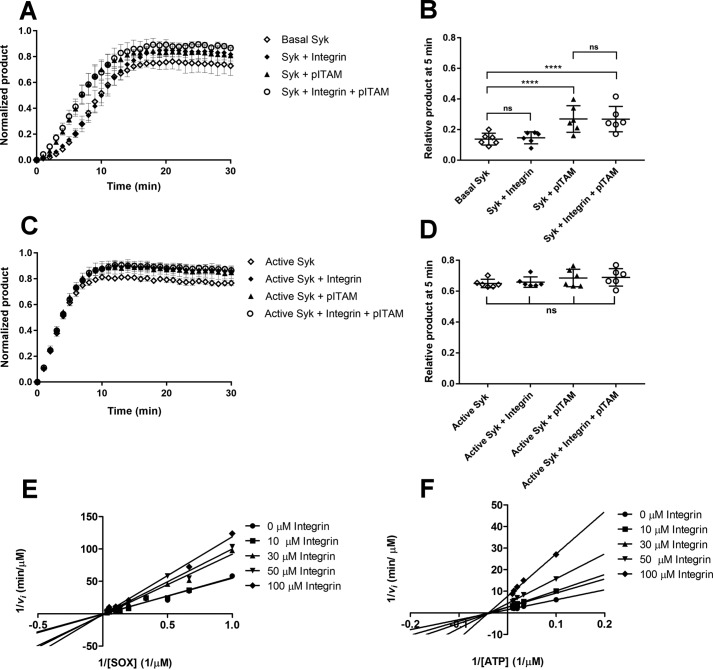
Figure 4.
Effect of pITAM and integrin β3 tail on Syk wildtype activity. A, kinetic measurement of Basal Syk in the absence of peptide (open diamonds), in presence of 30 μm integrin β3 cytoplasmic tail peptide (diamonds), 10 μm pITAM peptide (triangles), and a combination of both (open circles). The activity is expressed in terms of normalized product formation in time (min); basal Syk was used at concentration of 17 nm. Error bars correspond to the standard deviation calculated from six different experiments. B, ANOVA of the 5-min time point in the experiment shown in panel A. The data indicate that the presence of soluble pITAM activates the protein reducing the lag-phase of basal Syk. p value for basal Syk versus Syk + pITAM and basal Syk versus Syk + pITAM + integrin were both <0.0001. Comparing the basal Syk versus Syk + integrin and the Syk + pITAM versus Syk + integrin + pITAM with the ANOVA test resulted in nonsignificant (ns) p values thus showing that the soluble integrin β3 cytoplasmic tail peptide had no effect. C, kinetic measurement of active Syk in the absence of peptide (open diamonds), in the presence of 30 μm integrin β3 cytoplasmic tail peptide (diamonds), 10 μm pITAM peptide (triangles), and a combination of both (open circles). The activity is expressed in terms of normalized product formation in time (min). Active Syk was used at 17 nm. D, the ANOVA of the 5-min time point in panel C; it resulted in a nonsignificant (ns) p value suggesting that the pre-activated Syk is not modulated by the presence of either of two peptides. E and F, Lineweaver-Burk plots showing the competitive inhibition toward SOX peptide (E) and uncompetitive inhibition toward ATP (F) by the integrin β3 cytoplasmic tail. In each case 1/vi was plotted against 1/[SOX] (E) or 1/[ATP] (F). The experiments were carried out using active Syk at 4 nm.