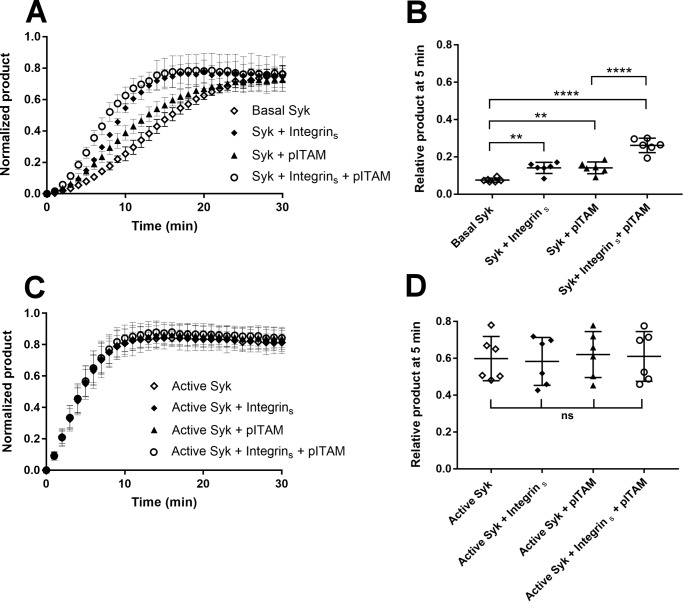
Figure 5.
Effect of clustered integrin β3 cytoplasmic tails on Syk activation. A, time course (min) of the normalized product formation in the absence of peptides (open diamonds) and in the presence of the integrin β3 cytoplasmic tail peptide coupled on the assay plate surface (diamonds), 10 μm pITAM (triangles), and combination of the two peptides (open circles). Basal Syk was used at 17 nm. Error bars correspond to the standard deviation calculated using six different experiments. B, ANOVA of the 5-min time point in panel A. The experiment shows that surface-bound integrin peptide reduces the lag phase of basal Syk; p value for basal Syk versus Syk + integrins was 0.0014 and was comparable with the p value for basal Syk versus Syk + pITAM, which was 0.0013. A combination of two peptides further accelerated the process (p value <0.0001). Integrin β3 cytoplasmic tail bound on the plate surface also has a synergic effect in the presence of pITAM peptide, in fact comparing the Syk + pITAM versus Syk + pITAM + integrins the ANOVA test gave a p value <0.0001. C, time course (min) of the normalized product formation in the absence of peptides (open diamonds) and presence of the integrin β3 cytoplasmic tail peptide coupled on the assay plate surface (diamonds), 10 μm pITAM (triangles), and a combination of two peptides (open circles). Active Syk was used at 17 nm. D, ANOVA of the experiment shown in panel C. p values were calculated using a ANOVA test including six separate experiments using the relative product formed at the 5-min time point as described under “Experimental procedures.” p values were nonsignificant (ns).