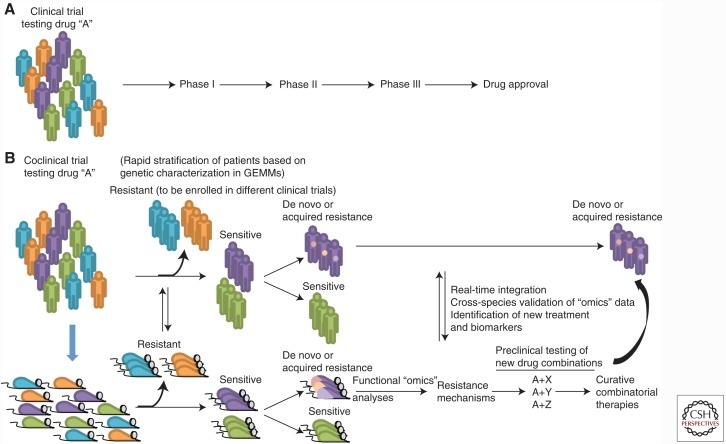
Figure 1.
The coclinical trial platform. (A) In the traditional clinical trial, little attention is paid to the molecular characteristics of the disease. When a minor fraction of patients are responsive, the overall response of the population might mask the responders. (B) In the coclinical trial project, relevant genetically engineered mouse models (GEMMs) and human patients are treated with the same drug and clinical protocol. Integrated analyses of data accrued in GEMMs and patients serve to stratify responsiveness and resistance on the basis of molecular and genetic criteria. Mechanisms underlying acquired resistance are also rapidly identified, and drug combinations to overcome such resistance are tested in GEMMs for their effectiveness.