Fig. 1. Biogenesis of hapalindole alkaloids.
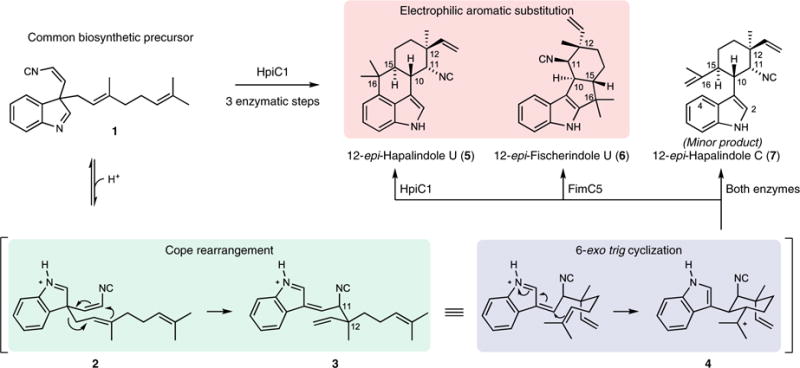
The hapalindole and fischerindole core ring systems arise from the common biosynthetic intermediate 1. Stig cyclases catalyze a Cope rearrangement and subsequent cyclization to generate tetracyclic products and trace levels of tricyclic shunt products. HpiC1 catalyzes formation of 5, while FimC5 catalyzes formation of 6, with identical stereochemistry at C11 and C12 but different C-ring regiochemistry.
