Fig. 3. Active site of SeMet HpiC1 W73M/K132M.
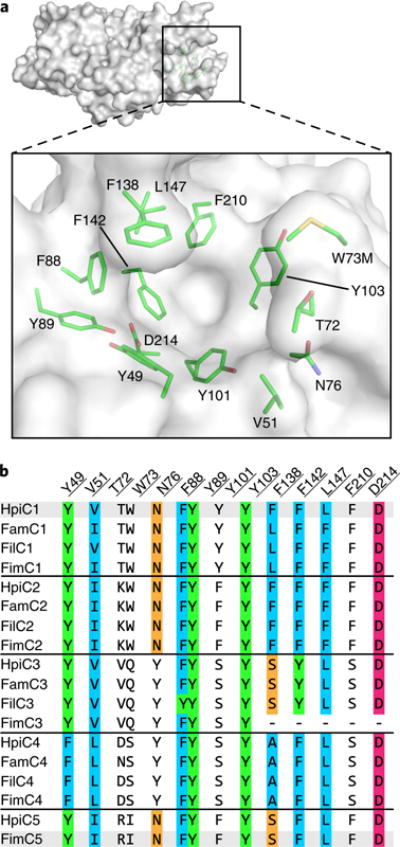
a, Surface representation of the SeMet HpiC1 active site. Key residues are shown as green sticks. Met73 is substituted for the native tryptophan residue. This mutant protein retained wild-type activity. b, Key active site residues shown in an alignment with those from other Stig cyclases. Residues are colored by conservation and side chain composition (ClustalX). The FimC3 gene product is truncated (dashes).
