Fig. 5. Quantum mechanics analysis.
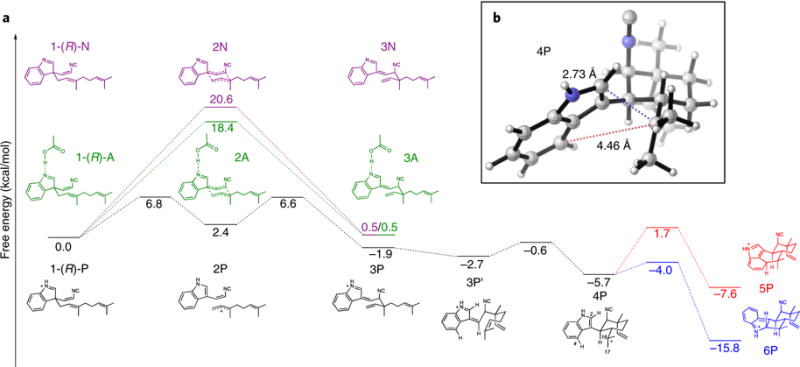
a, Cope rearrangement, 6-exo-trig cyclization, and electrophilic aromatic substitution cascade starting from the R enantiomer of substrate 1 in a near-attack conformation, leading to 5 precursor 5P and 6 precursor 6P. The energetics of the Cope rearrangement are computed with the neutral indolenine (pathway N), the N-protonated indolenine (pathway P), and the indolenine forming a hydrogen bond with acetic acid (pathway A). b, Optimized geometry of key intermediate 4P, which undergoes regioselective electrophilic aromatic substitution to form 5 or 6.
