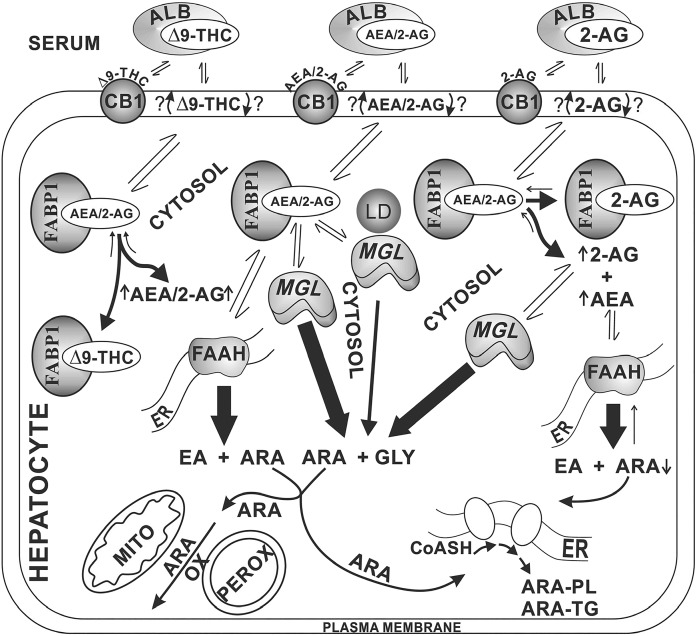
Fig. 8.
Proposed pathway regulating cultured primary mouse hepatocyte levels of ECs: impact of phytocannabinoids (Δ9-THC), 2-AG, and Fabp1 gene ablation. In serum, the highly lipophilic Δ9-THC, AEA, and 2-AG are bound by albumin (and lipoproteins) (34, 35, 61, 88, 89). After binding to hepatocyte CB1 and/or entering the plasma membrane (90), these ligands are translocated across the plasma membrane by an as yet unknown mechanism(s), with most evidence suggesting rapid spontaneous transbilayer migration (74, 75, 91). Due to their lipophilicity, these ligands require cytosolic binding/chaperone proteins for trafficking to intracellular sites of degradation/hydrolysis (21, 37, 79). In liver hepatocytes, the FABP1 is the most highly prevalent cytosolic lipophilic ligand binding/chaperone protein, recently shown to serve this function for phytocannabinoids, such as Δ9-THC, and ECs, such as AEA and 2-AG (19–21, 55). FABP1 then transports the bound AEA primarily to endoplasmic reticulum (ER) for hydrolysis by FAAH to yield ARA and ethanolamine (EA). Similarly, FABP1 also transports bound 2-AG for targeting to monoacylglycerol hydrolase (MGL), localized in cytosol and associated with lipid droplets (LD) to yield ARA and glycerol (GLY). The released ARA is then available for subsequent metabolism, i.e., oxidation (OX) [mitochondria (MITO), peroxisomes (PEROX)] or utilized for synthesis of ARA-containing phospholipids (PL) and triacylglycerols (TG). As shown on the left side of the figure, Δ9-THC competes with FABP1-bound AEA and 2-AG to displace them from the FABP1 binding site, thereby increasing hepatocyte AEA and 2-AG levels. As shown on the right side of the figure, 2-AG similarly competes with FABP1-bound AEA and 2-AG to displace them from the FABP1 binding site, thereby increasing hepatocyte AEA and 2-AG levels. Because 2-AG uptake is not driven by intracellular hydrolysis (77), continued uptake of 2-AG further exacerbates the hepatocyte level of 2-AG.