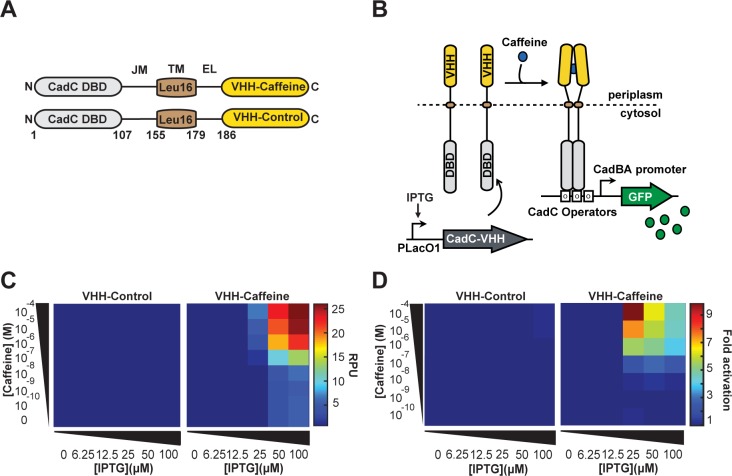
Figure 3.
A prokaryotic transmembrane receptor using a single-domain antibody for ligand detection. (A) General architecture of synthetic transmembrane receptor using the split-DBD principle. The DBD and Juxtamembrane of the CadC transcriptional activator were fused to an Leu(16)TM, an external linker, and VHH-Caffeine or VHH-Control as LBD. (B) Principle of transmembrane receptor activation. Genes encoding CadC-VHH fusions are placed under the control of the pLacO1 promoter. The N-terminal CadC DBD is located in the cytosol and the C-terminal VHH in the periplasm. In the presence of caffeine, the chimeric receptor CadC-VHH-Caffeine undergoes ligand-induced dimerization and activates downstream reporter gene expression. (C) Response of CadC-VHH-Caffeine and CadC-VHH-Control to increasing concentrations of caffeine at different expression level. (D) Activation fold of the two CadC-VHH fusions. For each IPTG concentration, fold changes were calculated from (C) as in Figure 1.