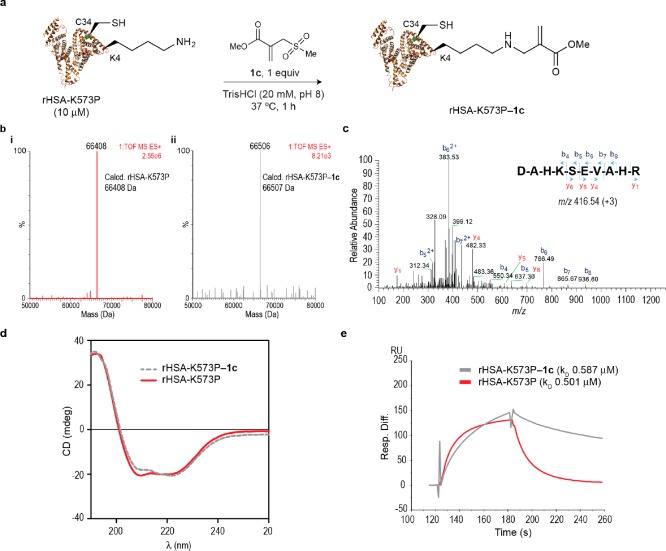
Figure 4.
Sequence-lysine conjugation effect of albumins on their binding affinity to human FcRn receptor. (a) Scheme for the bioconjugation reaction between mutant rHSA-K573P and sulfonyl acrylate 1c. General reaction conditions: rHSA-K573P was reacted with 1c (1 mol equiv) in TrisHCl (20 mM, pH 8.0) at 37 °C for 1 h. (b) Mass spectrometry characterization of lysine conjugation with 1c. ESI–MS spectra of rHSA-K573P (i) before (red) and (ii) after (gray) conjugation with 1c. (c) MS/MS spectrum of the m/z 416.54 triply charged ion of the lysine modified N-terminal peptide (1–10) DAHKSEVAHR. Modified residue underlined. (f) CD of rHSA-K573P and rHSA-K573P–1c. (d) CD analysis of the nonmodified rHSA-K573P and the conjugate rHSA-K573P–1c. (e) SPR comparison of the binding to human FcRn of rHSA-K573P and rHSA-K573P–1c.