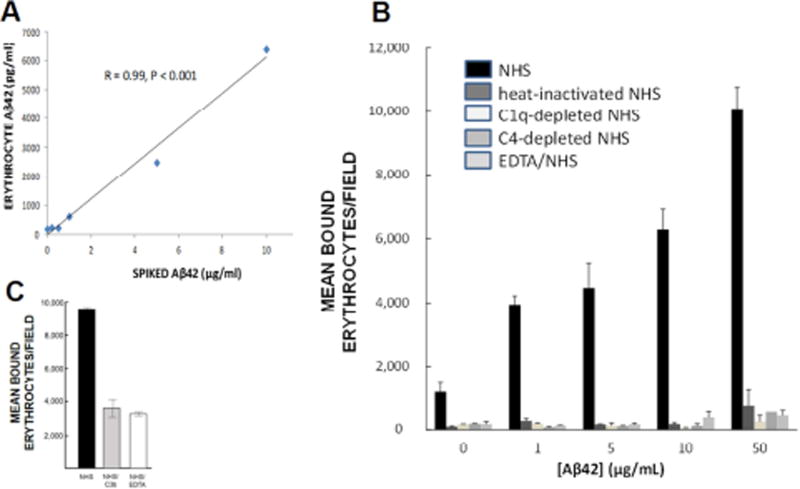
Fig. 4. Erythrocyte binding and capture of Aβ.
A) Following complement activation, Aβ is opsonized, tagging it for immune adherence reactions with erythrocytes. Here, NHS was incubated with various concentrations of Aβ42 to permit complement activation and opsonization, then incubated with erythrocytes from the same subject. Erythrocytes captured the Aβ42 in a significant dose-dependent manner. B) When Aβ42 was incubated with NHS, permitting complement activation and opsonization of the Aβ, significant dose-dependent binding to erythrocytes was observed (R = 0.91, P = 0.03). By contrast, binding was reduced to background with heat-inactivated NHS, which abolishes complement reactions. To control for the possibility that heat inactivation might also somehow inhibit non-complement-mediated erythrocyte capture of Aβ, we also included use of C1-depleted and C4-depleted NHS, which are specific to complement reactions, as well as EDTA treatment, a standard inhibitor of complement reactions. These conditions also abolished erythrocyte binding to Aβ, showing that complement mediation and formation of CR1 ligands is a primary mechanism for Aβ binding to erythrocytes. C) An excess of C3b, one of the ligands for CR1, was used to block erythrocyte C3b/CR1 binding sites, resulting in 62% inhibition of erythrocyte adhesion to Aβ42-coated plates.