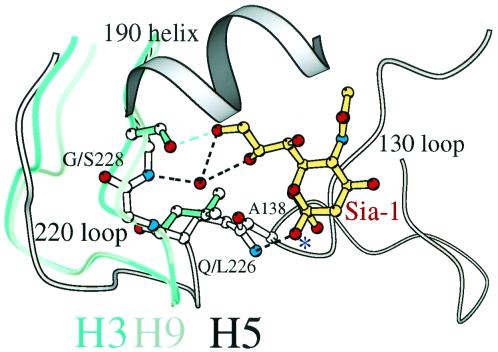
Figure 4.
Swine H9 and human H3 HA binding sites preferring α2,6 linkages are wider than avian H5 preferring α2,3. The H9 swine (gray), H3 human (green), and H5 avian (white) 226/228 loops are superimposed, showing that the H5 avian 220s loop (Gln-226/Gly-228) is closer to the opposing 130s loop than the H9 swine (Leu-226/Gly-228) or H3 human (Leu-226/Ser-228). Contact between Ala-138 and the lower methyl group of Leu-226 requires a more “open” site. The glycosidic oxygen of sialic acid (atom colors) is labeled with an asterisk. A water molecule (red sphere) mediates interactions between the amide group of Gly-228 and the 8- and 9-OHs of sialic acid in H9 swine and H5 avian HAs. The hydroxyl group of Ser-228 “replaces” the water molecule to form a hydrogen bond with 9-OH in the H3 human HA.