ABSTRACT
Salmonella spp. exhibit prolonged survivability and high tolerance to heat in low-moisture foods. The reported thermal resistance parameters of Salmonella spp. in low-moisture foods appear to be unpredictable due to various unknown factors. We report here that temperature-dependent water activity (aw, treatment temperature) plays an important role in the sharply increased thermal resistance of Salmonella enterica serovar Enteritidis PT 30 and its potential surrogate Enterococcus faecium NRRL B-2354. In our study, silicon dioxide granules, as carriers, were separately inoculated with these two microorganisms and were heated at 80°C with controlled relative humidity between 18 and 72% (resulting in corresponding aw,80°C values for bacteria between 0.18 and 0.72) in custom-designed test cells. The inactivation kinetics of both microorganisms fitted a log-linear model (R2, 0.83 to 0.97). Reductions in the aw,80°C values of bacterial cells exponentially increased the D80°C (the time needed to achieve a 1-log reduction in a bacterial population at 80°C) values for S. Enteritidis and E. faecium on silicon dioxide. The log-linear relationship between the D80°C values for each strain in silicon dioxide and its aw,80°C values was also verified for organic wheat flour. E. faecium showed consistently higher D80°C values than S. Enteritidis over the aw,80°C range tested. The estimated zaw (the change in aw,80°C needed to change D80°C by 1 log) values of S. Enteritidis and E. faecium were 0.31 and 0.28, respectively. This study provides insight into the interpretation of Salmonella thermal resistance that could guide the development and validation of thermal processing of low-moisture foods.
IMPORTANCE In this paper, we established that the thermal resistance of the pathogen S. Enteritidis and its surrogate Enterococcus faecium, as reflected by D values at 80°C, increases sharply with decreasing relative humidity in the environment. The log-linear relationship between the D80°C values of each strain in silicon dioxide and its aw,80°C values was also verified for organic wheat flour. The results provide new quantitative insight into the way in which the thermal resistance of microorganisms changes in low-moisture systems, and they should aid in the development of effective thermal treatment strategies for pathogen control in low-moisture foods.
KEYWORDS: Salmonella, Enterococcus faecium, water activity, silicon dioxide, thermal resistance, low-moisture food
INTRODUCTION
Recent Salmonella outbreaks in low-moisture foods (1–4) pose a serious food safety threat, with substantial economic impacts and social consequences, because many of those products are commonly consumed as ready-to-eat foods (e.g., nuts, cereals, pet foods) or are used as ingredients in a wide range of products (e.g., flours, spices, herbs). The presence of Salmonella bacteria in low-moisture foods is therefore identified as a potential hazard that requires preventive controls (5). Thermal processing is traditionally considered an effective method for the control of pathogens in high-moisture food products. However, Salmonella exhibits prolonged survivability and enhanced thermal resistance in a desiccated environment (6–9). A growing number of studies have investigated the effects of internal factors (e.g., Salmonella gene expression under desiccation stress) (10, 11) and external factors (e.g., food components, air humidity, temperature) on Salmonella thermal resistance in low-moisture foods. Recent findings suggest that temperature-dependent water activity (aw, treatment temperature), defined as the ratio of the water vapor pressure of a food system to that of pure water at the treatment temperature, plays a key role in altering bacterial thermal resistance (12, 13).
Unlike the widely used aw,20–25°C (measured by an aw meter at room temperature prior to thermal treatment), as reported in the current literature, aw, treatment temperature is the real-time aw during thermal processing. It is well known that aw of a food matrix is greatly influenced by food composition (12) and temperature (14, 15). At high temperatures, food components either release or absorb water molecules, effects that can be measured by changes in the relative humidity of the headspace in the food system (16–18). A previous study (12) considered that aw, treatment temperature partially contributes to the vast difference between the D80°C (the time needed to achieve a 1-log reduction in the bacterial population at 80°C) values for Salmonella enterica serovar Enteritidis PT 30 in peanut butter (17 min) and in all-purpose wheat flour (6.9 min). Prior to this study, the influence of aw, treatment temperature on the thermal resistance of microorganisms had not been reported.
Enterococcus faecium NRRL B-2354 has been widely evaluated as a potential Salmonella surrogate in the thermal processing of low-moisture foods; it has consistently shown greater thermal resistance than Salmonella in different low-moisture foods (19–21). As such, it has been utilized to validate different thermal-processing technologies (e.g., air moisture, roasting, infrared heating) (19, 22, 23). However, studies comparing the thermal resistances of Salmonella and E. faecium were limited to several selected food systems. No previous studies that support the general utility of E. faecium as a Salmonella surrogate in thermal processes have been reported (25). It is, therefore, desirable to evaluate the thermal resistance of each of these species in a low-moisture environment independently of food components.
The aw of a food product changes with temperature (12, 14, 24). The degree of change is influenced by different factors, including moisture content, food composition, and temperature (13, 14). In a closed system, food moisture content remains constant. The aw of the food sample at a specific temperature (e.g., 80°C in this study) is influenced mainly by food composition (25). Due to the limited temperature range of commercial aw meters (20 to 60°C), most published studies have failed to estimate the aw of food matrices at temperatures above 60°C (13). Recently, a thermal cell installed with high-temperature relative humidity sensors was designed (12) and modified (25) to measure the aw of foods above 60°C. The aw,80°C data of peanut butter, all-purpose flour, wheat flour, almond meal, and nonfat milk powder were reported (12, 25). The Clausius-Clapeyron equation (CCE) has been applied to enable prediction of the aw at elevated temperatures for wheat flour, almond flour, and nonfat milk powder (25).
In the present study, we aimed to investigate the influence of aw,80°C on the thermal resistance parameter D80°C for S. Enteritidis and E. faecium. We chose S. Enteritidis as the target pathogen because it is primarily responsible for outbreaks in ready-to-eat nuts and nut spreads (19) and has been reported to exhibit high thermal resistance in low-moisture foods (20, 26, 27). We studied silicon dioxide (SiO2) as a low-moisture carrier and used lithium chloride (LiCl; 0 to 18 mol/kg) to control relative humidity, keeping it between 18 and 72% (corresponding to an aw,80°C for bacterial cells between 0.18 and 0.72) in the headspace of a custom-designed thermal water activity cell (TAC) (28) during the isothermal inactivation tests. Finally, we compared the D80°C values of S. Enteritidis and E. faecium in SiO2 (at a controlled aw,80°C) with those in wheat flour (at low but uncontrolled aw,80°C levels). The specific objectives of this study were (i) to characterize SiO2 as a carrier for S. Enteritidis and E. faecium, (ii) to evaluate the thermal inactivation of both strains at a constant aw,80°C in TACs, (iii) to model and compare the relationships between logD80°C and aw,80°C values for both strains, and (iv) to verify the relationships by examining the reported D80°C values of these strains in wheat flour and the estimated aw,80°C of wheat flour.
RESULTS
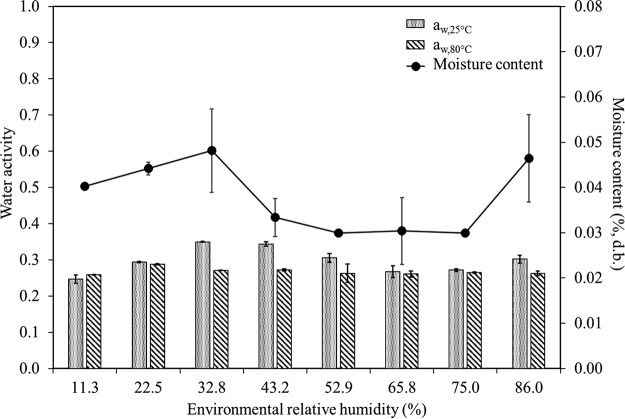
The water activities at 25 and 80°C of SiO2 that was preconditioned over a wide range of relative humidity at 25°C ranged from 0.25 to 0.31 (Fig. 1). The moisture content stayed consistently low (0.03 to 0.06%, dry basis). The variation in the relative humidity level at which SiO2 was preconditioned did not result in changes in the moisture content or the aw of the SiO2 samples.
FIG 1.
Water activity levels measured at 25°C (0.31 ± 0.05) and 80°C (0.27 ± 0.01) and corresponding water content (0.04% ± 0.01%, dry basis [d.b.]) of silicon dioxide preconditioned (n = 2) at different relative humidity levels (generated by saturated salt solutions). Results of two replicated experiments (n = 2) are shown.
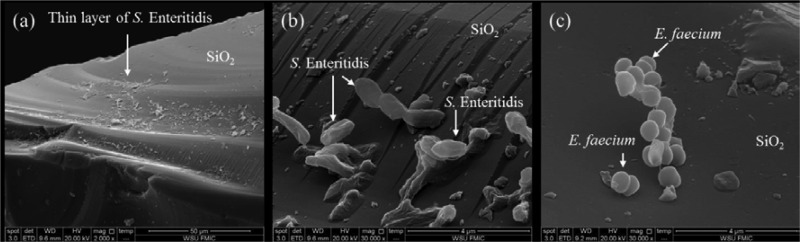
The desiccation of the culture on SiO2 caused a ∼2-log CFU/g reduction in the bacterial cells. The initial inoculation level of SiO2 for both strains was 7.8 ± 0.2 log CFU/g. Scanning electron microscopy (SEM) images of S. Enteritidis and E. faecium inoculated onto SiO2 are shown in Fig. 2. Bacterial cells spread on SiO2 particles that had flat surfaces. S. Enteritidis cells were elongated and were closely attached to SiO2 granules, while E. faecium cells were clustered and maintained their coccus shape. Both strains were fully exposed to the surrounding air.
FIG 2.
(a) SiO2 serves as a vehicle for carrying S. Enteritidis cells on its surface. (b) S. Enteritidis cells shrank significantly into a flat form with many wrinkles. (c) E. faecium cells maintained their shapes as cocci.
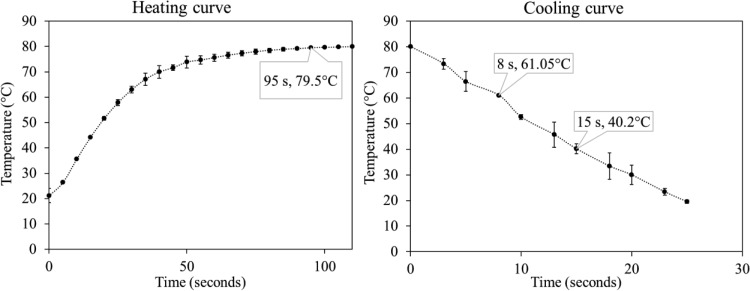
Temperature changes in the geometric center of SiO2 in an oil bath at 80°C are shown in Fig. 3. The 1.0-g SiO2 samples were heated to 79.5°C within 95 s and were cooled from 80°C to below 40°C within 15 s in an ice water bath. The rapid heating and cooling (2.67°C/s) of SiO2 samples in TACs indicate efficient heat transfer in the isothermal treatments.
FIG 3.
Heating and cooling curves of the centers of silicon dioxide samples in thermal-water activity cells. Results of two experiments (n = 2) are shown.
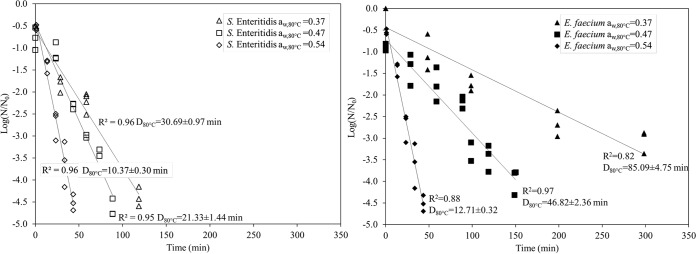
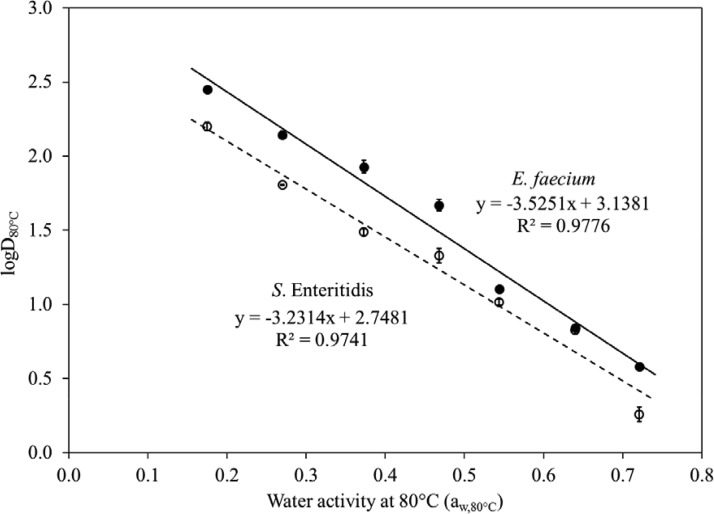
The survival curves of both bacteria fitted the log-linear model well (R2, 0.82 to 0.97 [Table 1 and Fig. 4]). Over a wide range of aw,80°C values, D80°C values in this study differed >80-fold, from 2.14 ± 0.16 min (for S. Enteritidis at an aw,80°C of 0.72) to 170.98 ± 18.54 min (for S. Enteritidis at an aw,80°C of 0.18). The logD80°C values of both strains as a function of aw,80°C, from 0.18 to 0.72, fitted a linear trend (R2, 0.97 to 0.99) (Fig. 5). Based on equation 5, the zaw (the change in aw,80°C needed to change D80°C by 1 log) values of S. Enteritidis and E. faecium were 0.31 and 0.28, respectively.
TABLE 1.
Parameter estimates (± standard errors) for the primary linear model, root mean squared error, and R2
| Strain | aw,25°C ± 0.02 | aw,80°C (predicted) | D80°C (min) | RMSEa (log CFU) | R2 |
|---|---|---|---|---|---|
| S. Enteritidis | 0.11 | 0.18 | 159.31 ± 5.77 | 0.26 | 0.93 |
| 0.20 | 0.27 | 64.04 ± 0.16 | 0.58 | 0.88 | |
| 0.31 | 0.37 | 30.69 ± 0.97 | 0.27 | 0.96 | |
| 0.42 | 0.47 | 21.33 ± 1.44 | 0.48 | 0.91 | |
| 0.50 | 0.54 | 10.37 ± 0.30 | 0.29 | 0.97 | |
| 0.61 | 0.64 | 6.80 ± 0.25 | 0.42 | 0.89 | |
| 0.70 | 0.72 | 1.80 ± 0.12 | 0.47 | 0.83 | |
| E. faecium | 0.11 | 0.18 | 281.78 ± 5.78 | 0.32 | 0.97 |
| 0.20 | 0.27 | 139.16 ± 5.07 | 0.57 | 0.90 | |
| 0.31 | 0.37 | 85.09 ± 4.75 | 0.49 | 0.82 | |
| 0.42 | 0.47 | 46.82 ± 2.36 | 0.21 | 0.97 | |
| 0.50 | 0.54 | 12.71 ± 0.32 | 0.53 | 0.88 | |
| 0.61 | 0.64 | 6.97 ± 0.06 | 0.29 | 0.95 | |
| 0.70 | 0.72 | 3.81 ± 0.11 | 0.41 | 0.89 |
RMSE, root mean squared error.
FIG 4.
Representative survival curves of Salmonella Enteritidis and E. faecium in SiO2 at 80°C with a water activity of 0.37, 0.47, or 0.54 predicted at 80°C and their trend lines, R2, and estimated D80°C values. Experiments were repeated three times independently.
FIG 5.
LogD80°C (decimal reduction time to achieve 90% population reduction at 80°C) values for S. Enteritidis and E. faecium in SiO2 increased with decreasing water activity levels at 80°C (R2 = 0.98).
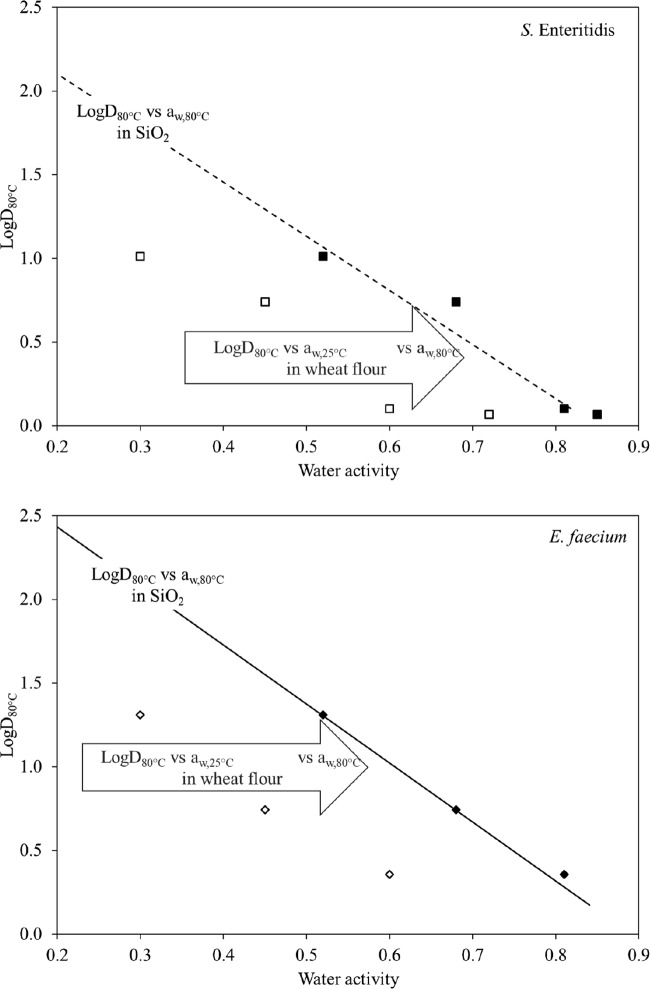
The estimated aw,80°C values of wheat flour ranged from 0.52 to 0.85, consistently higher than the corresponding aw,25°C (Table 2). The logD80°C values for S. Enteritidis and E. faecium in wheat flour as a function of aw,25°C (open symbols) and aw,80°C (filled symbols) are shown in Fig. 6. The filled symbols overlap with linear trend lines in Fig. 6, which were previously obtained from linear least regressions of the same strains in SiO2 (equation 5).
TABLE 2.
D80°C of S. Enteritidis and E. faecium in wheat flour reported for different aw,25°C values in the literature and the corresponding aw,80°C estimated in this study
| Strain | aw,25°C ± 0.02 | aw,80°C ± 0.02a | Δaw ± 0.02 | D80°C (min) | Reference for D80°C |
|---|---|---|---|---|---|
| S. Enteritidis | 0.31 | 0.52 | 0.21 | 10.27 ± 0.65 | 26 |
| 0.43 | 0.68 | 0.25 | 5.51 ± 0.22 | ||
| 0.58 | 0.81 | 0.23 | 1.27 ± 0.06 | ||
| 0.70 | 0.85 | 0.15 | 1.17 ± 0.06 | ||
| E. faecium | 0.30 | 0.52 | 0.22 | 20.4 ± 0.85 | 45 |
| 0.45 | 0.68 | 0.23 | 5.56 ± 0.49 | ||
| 0.60 | 0.81 | 0.21 | 2.27 ± 0.25 |
Predicted aw,80°C values of wheat flour were determined by the Clausius-Clapeyron equation (25).
FIG 6.
LogD80°C values for S. Enteritidis (top) and E. faecium (bottom) plotted against aw at 25°C (open symbols) or aw at 80°C (filled symbols) in wheat flour. In both panels, the lines show the exponential trends for S. Enteritidis or E. faecium in SiO2 (Fig. 5).
DISCUSSION
Characterization of SiO2 (uninoculated and inoculated) (Fig. 1).
The relatively flat curves of aw over a wide range of environmental relative humidities indicate that SiO2 neither absorbs nor releases moisture as the environmental humidity changes. SiO2 has been utilized by other researchers as a carrier coated with chemicals (29) or as a foundation for biosensors used in bacterial detection (30). In addition, SiO2 is autoclavable and has not been reported to have any antibacterial effects. Thus, SiO2 is a good carrier material for bacterial inactivation studies in low-moisture foods.
The thin layers of bacterial cells on SiO2 surfaces in the SEM images support our assumption that bacterial cells in SiO2 are directly exposed to the ambient environment. Syamaladevi et al. (31) estimated that it takes no more than a second for a bacterial cell to adjust its water content in response to humidity changes in the environment. Thus, bacterial cells were expected to be inactivated under isothermal and iso-aw,80°C conditions in TACs.
With low moisture contents (0.03 to 0.06%, dry basis), SiO2 allows bacterial cells to be dehydrated naturally. The rate of dehydration depends on environmental relative humidity. S. Enteritidis and E. faecium survivors in SiO2 are assumed to represent typical Salmonella and E. faecium cells in low-moisture environments.
Interpretation of TAC data.
The purpose of using TACs was to expose the inoculated bacterial cells to controlled relative humidities at 80°C. When opening the TACs at room temperature, we observed slight water condensation in some of the TACs after thermal treatments at an aw,80°C of ≥0.50. We were concerned that if bacterial cells absorbed water droplets at a lethal temperature (e.g., >60°C), they might be inactivated faster than if they were in low-moisture environments (13). However, based on the temperature profiles shown in Fig. 3, only 15 s was required for the SiO2 samples in a TAC to cool from 80°C to 40°C. We anticipate that in such a short time, the water condensation in TACs would not alter inactivation results.
The aw,80°C values used in this study were estimated from water vapor pressure generated from LiCl solutions of different molalities. Water vapor pressure can also be measured as relative humidity (expressed as a percentage) and converted into aw (aw = relative humidity/100). In the present study, aw,80°C refers to the equilibrium aw of the bacterial cells when exposed to a specific relative humidity at 80°C. Our recent study (31) suggests that, through moisture diffusion, the extremely small size of bacterial cells allows them to adjust the intracellular moisture content according to changes in environmental relative humidity within seconds. The aw,80°C was used for interpretation and data analysis because aw,80°C can be easily related to aw,25°C, which has been used to define low-moisture foods (32) and has been widely studied for its impact on thermal resistance of microorganisms in low-moisture environments. On the other hand, relative humidity (calculated as 100 × aw) can be used as an independent parameter for process control in an open system, such as baking, roasting, or hot-air drying. The D80°C values in Fig. 5 and 6 can be interpreted as functions of relative humidity at high temperatures and used to develop process conditions for effective control of pathogens. On the basis of these figures, it is clear that high humidity in an open thermal treatment could facilitate pathogen control in low-moisture foods.
Thermal resistance of S. Enteritidis and E. faecium.
E. faecium had consistently higher D80°C values than S. Enteritidis under the same test conditions. This observation matches data reported for E. faecium and Salmonella in almond kernels (20), balanced carbohydrate-protein meals (33), and wheat flour (34). The zaw (as a function of aw,80°C) of E. faecium, 0.28, is statistically significantly different from that of S. Enteritidis, 0.31 (P < 0.05). This observed difference lies within the accuracy range of our aw meters (±0.02). The results presented above suggest that E. faecium is an appropriate surrogate for Salmonella in thermal processes at 80°C.
Thermal resistance of microorganisms as influenced by aw,80°C.
For both S. Enteritidis and E. faecium, a reduced aw,80°C increased D80°C exponentially (R2 = 0.98).
Thermal inactivation of bacterial cells might be due to the loss of functions of subsets of heat-sensitive proteins (37), which may be denatured at elevated temperatures. Water loss from bacterial cells at low aw results in more-stable protein structures and hinders thermal denaturation. This has been considered a major reason for the high thermal resistance of bacterial spores. For example, Sunde et al. (38) reported a low water rotational correlation time (5 × 10−11 s) in the dense core of Bacillus subtilis and concluded that most proteins in B. subtilis spores are rotationally immobilized. The similar mechanism might explain the increased thermal resistance of S. Enteritidis and E. faecium at reduced aw.
Although S. Enteritidis and E. faecium were heat treated on SiO2 granules in this study, the results may provide important insight into how bacteria in different food matrices would respond to heat treatments.
Verifying the log-linear model of D80°C and aw,80°C.
Wheat flour was chosen as the food system for verification of the log-linear relationship shown in Fig. 5. At fixed moisture contents, the aw,80°C values of wheat flour samples were higher than their aw,25°C values (Table 2). At a high moisture content (corresponding to an aw,25°C of 0.7), the Δaw of wheat flour was smaller (0.15) than that at lower aw,25°C levels (Δaw, 0.21 to 0.25). Thus, the logD80°C values of S. Enteritidis and E. faecium in wheat flour shifted to the right and matched the trend lines describing the logD80°C of the same strains in SiO2 as a function of aw,80°C (Fig. 6). The linear relationships between the logD80°C values of S. Enteritidis and E. faecium and the aw,80°C value of wheat flour had R2 values of 0.95 and 0.99, respectively. This trend confirmed our observation in Fig. 5 that aw, treatment temperature influenced the thermal resistance of microorganisms in low-moisture foods in a log-linear fashion.
Wheat flour and SiO2 represent different low-moisture matrices. Yet, at the same aw,80°C, the logD80°C values with the two substrates overlapped both strains. Therefore, the logD80°C of microorganisms appears to be closely related to the aw,80°C of bacterial cells independent of the low-moisture systems.
Relevance of this study to food safety in low-moisture foods. (i) E. faecium as a Salmonella surrogate in thermal processes for low-moisture foods.
To date, data in support of the use of E. faecium as a Salmonella surrogate in validating the thermal processing of various low-moisture foods (20, 33, 39) are insufficient. The protocols and guidelines established for the use of E. faecium are limited to a small number of products and specific process conditions (19). In the present study, side-by-side comparison between E. faecium and S. Enteritidis was conducted independently of food matrices (in SiO2). The results proved that E. faecium is more heat resistant (higher D80°C) than S. Enteritidis at any given aw,80°C between 0.18 and 0.72. Our study indicates that E. faecium can be a valid Salmonella surrogate in the thermal processing of low-moisture foods over a wide range of water activities.
(ii) Calibration of the surrogate E. faecium prior to industrial application.
Our study aims to contribute to establishing standard protocols for surrogate validation and calibration and for further mathematic modeling of the thermal resistance of microorganisms in low-moisture foods during heat treatments. In practice, depending on experimental procedures and methods of culturing and collecting bacterial cells, not every batch of E. faecium would perform as strongly as expected. It is critical to determine the heat resistance of a surrogate prior to any thermal process validations. The guideline published by the Almond Board of California provides a “heat resistance test” procedure to characterize the resistance of E. faecium in almonds (19). However, the equipment (metal mesh and convection/forced-air oven) is not necessarily applicable to other food matrices, such as powdered foods. This guideline does not specify the need for monitoring the relative humidity or temperature of the food matrix, which are proven to be key factors (12). The lack of these parameters in a heat resistance test would lead to significant variance depending on the real-time relative humidity of the air. Moreover, the acceptable heat resistance range (≤2.5 log reduction at 280°F for 15 min) may not apply to other low-moisture foods.
This study provides another approach for calibrating a surrogate in low-moisture foods with less uncertainty by fixing the temperature (80°C) and aw,80°C using TACs. For any batch of E. faecium, the D80°C values provided as a function of aw,80°C in Table 1 could be used as calibration references. For instance, the D80°C value for E. faecium at an aw,80°C value of 0.72 is 3.81 ± 0.11 min. The acceptable Salmonella surrogate should undergo a ≤2.5-log reduction at 80°C, with an aw,80°C of 0.72, in 9.53 min (3.81 × 2.5 = 9.53).
(iii) Mathematic modeling of thermal resistance parameters of Salmonella spp. and E. faecium in low-moisture foods under various conditions.
A modified Bigelow-type model was suggested to predict D values by the following equation 1 (40):
| (1) |
where Dref is the D value (in minutes) at Tref and aw,ref; Tref and aw,ref are a reference temperature and aw, respectively; and zT is the temperature needed to change the D value 10-fold (in degrees Celsius). Based on the present study, it is appropriate to use aw, treatment temperature instead of aw at room temperature in the model presented above. The value of the aw, treatment temperature of a food product can be provided by mathematic estimation in a closed system (12, 25) or by the relative humidity of the environment in an open system.
Our data support increasing aw, treatment temperature in the thermal processing of low-moisture foods to achieve easier kill of pathogens. However, these D80°C values may not apply to open systems with changing water vapor pressures surrounding bacterial cells.
MATERIALS AND METHODS
S. Enteritidis and E. faecium were acquired from Linda Harris at the University of California, Davis. Both strains were kept at −80°C in tryptic soy broth (TSB) supplemented with 20% (vol/vol) glycerol. Tryptic soy agar (TSA), yeast extract, and peptone water were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ); ammonium iron(III) citrate was purchased from Sigma-Aldrich Corporation (St. Louis, MO); and sodium thiosulfate 5-hydrate was made by J. T. Baker (Avantor Performance Materials, Center Valley, PA). Silicon dioxide (SiO2; 0.2- to 0.7-mm granules) was purchased from Umicore, Brussels, Belgium. LiCl was purchased from J. T. Baker (Avantor Performance Materials, Center Valley, PA). An AquaLab vapor sorption analyzer (VSA) was purchased from METER Group Inc. (formerly Decagon Devices, Inc., Pullman, WA). A vacuum oven (Yamato Scientific America Inc., CA) was used to dry SiO2 to an aw of <0.1. An oil bath (GP-400; Neslab, Newington, NH) filled with ethylene glycol (VWR International, Radnor, PA) was used for isothermal treatments. We used custom-designed aluminum thermal water activity cells (TACs) (28) to assess the thermal inactivation studies (see Fig. S1 in the supplemental material).
Characterization of SiO2.
Water equilibrium isotherms of SiO2 particles were generated in order to understand how SiO2 exchanges moisture with the environment. In the generation of adsorption isotherms of SiO2 at 80°C, SiO2 samples were first vacuum-dried overnight at an absolute pressure of 10 kPa inside the vacuum oven at 50°C and then preconditioned in air-tight containers containing different saturated salt solutions at 23°C for 2 weeks. The saturated salt solutions (with the corresponding relative humidity at saturated status in parentheses) were LiCl (11.3%), CH3COOK (22.5%), MgCl2 (32.8%), K2CO3 (43.2%), MgNO3 (52.9%), NaNO2 (65.8%), NaCl (75.3%), and KCl (84.3%) at 23°C (Fisher Scientific, Houston, TX) (41). After equilibration, an aluminum cell (inner diameter, 42 mm; height, 19 mm) with a relative humidity sensor (HX15-W; Omega Engineering, Inc., Stamford, CT) was used to measure the aw,80°C (12). The equilibrated SiO2 (2 to 3 g) was loaded and sealed in the aluminum cell mentioned above and was transferred to a forced-air convection oven at 80°C; aw,80°C was calculated and was displayed for recording. Then the sealed cell was removed from the oven and was kept at room temperature for ∼30 min to reach the ambient temperature (∼23°C). The moisture contents of the equilibrated samples were obtained by heating a small amount (3 to 5 g) at 80°C for 10 h in the vacuum oven under an absolute pressure of 10 kPa.
To generate desorption isotherms at 80°C, SiO2 was mixed with distilled water to achieve an aw,25°C of ∼1.0. The samples were then equilibrated in tightly closed jars containing saturated salt solutions. The aw,80°C of all preconditioned samples was measured in aluminum cells with relative humidity sensors as described above.
Preparation of inoculated SiO2.
A bacterial inoculum of S. Enteritidis or E. faecium was prepared according to the procedures described by Hildebrandt et al. (42). Briefly, frozen S. Enteritidis or E. faecium was subjected to two consecutive transfers (24 h each at 37°C) in 9 ml of tryptic soy broth supplemented with 0.6% (wt/vol) yeast extract (TSBYE), and then 1 ml was evenly spread onto a plate (150 by 15 mm) of tryptic soy agar supplemented with 0.6% (wt/vol) yeast extract (TSAYE). The bacterial lawn was harvested into 5 ml of sterile 0.1% peptone water.
One milliliter of each inoculum was mixed with 100 g SiO2 (previously autoclaved to remove background bacteria) in a sterile stomacher bag until the pellet was visibly mixed. After mixing, the inoculated SiO2 was placed in 150-mm-diameter petri dishes (without lids) and was dried rapidly under a biosafety hood for 12 h at the ambient temperature (∼23°C) with the fan running. Ten 1-g samples were randomly selected and were analyzed to confirm the uniformity of inoculum distribution. All steps were repeated three times as independent biological replicates of both strains.
SEM imaging of microorganisms inoculated in SiO2.
A small amount of SiO2 particles inoculated with either S. Enteritidis or E. faecium was fixed in 2% (vol/vol) paraformaldehyde–2% (vol/vol) glutaraldehyde–0.1 M phosphate buffer overnight at 4°C and was then rinsed in several changes of deionized water. The samples were rapidly frozen (∼2 min) in liquid nitrogen and were then freeze-dried at −45°C overnight (Flexi-Dry freeze dryer; SP Scientific, Gardiner, NY). Freeze-dried SiO2 granules were thinly spread onto double-coated carbon conductive tabs (Ted Pella Inc., Redding, CA) and were gold coated in a vacuum evaporator (Technics Hummer V sputter coater; Technic, San Jose, CA) to a thickness of 6 nm. An environmental field emission gun scanning electron microscope (SEM) (Quanta 200F; FEI Company, Hillsboro, OR) was used to examine samples, and the images were captured by a digital camera (Quartz Imaging Corporation, Vancouver, British Columbia, Canada).
Control of relative humidity (aw,80°C of bacterial cells) in TACs.
An LiCl solution was selected to control the relative humidity of the headspace in TACs because LiCl generates specific water vapor pressure depending on its concentration and temperature. The molalities of LiCl solutions and the corresponding water vapor pressures at temperatures between 20 and 100°C have been reported by Gibbard and Scatchard (43). A user-friendly chart relating relative humidity and LiCl solutions in an article by Tadapaneni et al. (28) was used to select the appropriate LiCl molality for the desired relative humidity at 25 and 80°C in TACs. In our study, we used 6.1- to 18-mol/kg LiCl solutions to provide relative humidities of 18 to 72% at 10% intervals. Since the relative humidity (expressed as a percentage) in the headspace of the TAC is directly related to the aw,80°C (calculated as relative humidity/100) of the LiCl solution, we use only aw,80°C values in most of the discussion.
The estimated aw,80°C values during the isothermal inactivation of microorganisms on SiO2 were 0.18, 0.27, 0.37, 0.47, 0.55, 0.63, and 0.72 (see Table S1 in the supplemental material). The aw,80°C levels of 0.8 and 0.9 were not tested in this study because both microorganisms were inactivated by more than 3 log units after the come-up time (CUT) at these two aw levels, and the D80°C values of both strains were too low to be determined.
Inactivation of microorganisms in TACs.
With prepared LiCl salt solutions, three portions of 1 g inoculated SiO2 were placed in thin aluminum cups (Fig. S1b in the supplemental material). The cups (∼0.2 g; thickness, 0.02 mm) were handmade in the shape of TAC sample wells from heavy-duty aluminum foil (Western Family Foods, Tigard, OR). Three cups were placed in one TAC base with 3 ml of the respective LiCl solution in the central liquid-holding well. The TACs were then sealed and were stabilized at room temperature (∼23°C) for >2 h to let the water vapor pressure from the LiCl solution reach an equilibrium inside the TACs. Isothermal inactivation tests were performed at 80°C for seven aw,80°C levels (0.18 to 0.72, with intervals of 0.1).
To obtain thermal death curves for S. Enteritidis and E. faecium, TACs containing inoculated samples at selected aw,80°C values (controlled by the LiCl solution in the central well) were subjected to isothermal treatments at 80°C. Only two TACs at one time were placed in the oil bath containing the ethylene glycol heating medium so as to avoid an excessive temperature drop. The CUT for the geometric center of the sample to reach 79.5°C was 1.58 min. Thermal treatments at uniform time intervals were performed, starting with time zero (at the CUT). Once removed from the oil bath, TACs were immediately placed in an ice water bath to stop thermal inactivation. Sample cups filled with 1 g treated SiO2 were transferred gently from the TACs for the recovery and enumeration of survivors (Fig. S1c in the supplemental material).
Thermally treated SiO2 samples were transferred to sterile stomacher bags, diluted 1:9 with 0.1% peptone water, and homogenized for 3 min at 260 rpm in a Seward stomacher (Seward, London, UK) (44). Appropriate serial dilutions were plated in duplicate onto modified TSAYE with 0.03% sodium thiosulfate and 0.05% ammonium ferric citrate (for S. Enteritidis) or TSA (for E. faecium). Colonies were enumerated after incubation at 37°C for 48 h. All of the tests were conducted one time per independent biological replicate.
Model verification using reported and estimated data.
To verify the relationships between the logD80°C and aw,80°C values of S. Enteritidis and E. faecium in SiO2, data reported on thermal resistance parameters and water sorption isotherms in wheat flour (Eden Foods, Clinton, MI) were analyzed. The D80°C values of S. Enteritidis and E. faecium in wheat flour were reported by Smith et al. (26) and Liu et al. (45). In those studies, though, sample water activities at 25°C were reported.
The aw,80°C values of the wheat flour samples were estimated from the reported aw,25°C values by CCEs according to reference 25, as follows:
| (2) |
where aw1 and aw2 are the aw values of a sample with a fixed moisture content at temperatures T1 and T2, respectively; qst is the net isosteric heat of sorption (in joules per mole) that describes the difference between the total enthalpy change for the sorption process and the latent heat of water vaporization; and R is the universal gas constant (8.314 J mol−1 K−1).
For wheat flour, qst was calculated (25). The qst is dependent on the moisture content of the food matrix and the relationship as shown in equation 3:
| (3) |
The differences between the estimated aw,80°C and corresponding aw,25°C values were obtained as Δaw values.
Data analysis.
The first-order kinetic, or log-linear, model has been primarily used to characterize bacterial inactivation (46) as follows:
| (4) |
where log reduction, log(N/N0), was calculated by dividing survivor counts (N) by the population at time zero (N0) for the respective replicate; t is the time of the isothermal treatment (in minutes) after the CUT, and D is the time (in minutes) required to reduce the microbial population by 90% at a specified temperature (in degrees Celsius).
The relationships between the logD80°C values of S. Enteritidis and E. faecium and the aw,80°C values in this study were modeled according to reference 47 as follows:
| (5) |
where A is constant and zaw describes the water activity change necessary to alter the thermal death time by 1 log-cycle.
All water activities were estimated at 80°C (Table S1 in the supplemental material). The graphs were drawn with Excel or PowerPoint 2016, and tables were made with Excel 2016. All differences were considered significant if the probability was <0.05. Analysis of variance (ANOVA) and t tests for the experimental data were performed using Minitab 14.1.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the USDA Agricultural and Food Research Initiative (AFRI), Coordinated Agricultural Projects (CAP) (grant 2015-68023-23415). We also thank the China Scholarship Council for granting a scholarship to Shuxiang Liu for her Ph.D. study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02742-17.
REFERENCES
- 1.FDA. 15 January 2016. FDA investigated multistate outbreak of Salmonella infections linked to raw nut butter products. FDA, Silver Spring, MD: https://www.fda.gov/food/recallsoutbreaksemergencies/outbreaks/ucm475416.htm. [Google Scholar]
- 2.Isaacs S, Aramini J, Ciebin B, Farrar J, Ahmed R, Middleton D, Chandran A, Harris L, Howes M, Chan E. 2005. An international outbreak of salmonellosis associated with raw almonds contaminated with a rare phage type of Salmonella Enteritidis. J Food Prot 68:191–198. doi: 10.4315/0362-028X-68.1.191. [DOI] [PubMed] [Google Scholar]
- 3.CDC. 15 January 2016. Multistate outbreak of Salmonella Paratyphi B variant L(+) tartrate(+) infections linked to JEM raw brand sprouted nut butter spreads (final update). CDC, Atlanta, GA: https://www.cdc.gov/salmonella/paratyphi-b-12-15/index.html. [Google Scholar]
- 4.CDC. 20 May 2016. Multistate outbreak of Salmonella Montevideo and Salmonella Senftenberg infections linked to Wonderful pistachios (final update). CDC, Atlanta, GA: https://www.cdc.gov/salmonella/montevideo-03-16/index.html. [Google Scholar]
- 5.FDA. 2015. FSMA final rule for preventive controls for human food. FDA, Silver Spring, MD: https://www.fda.gov/food/guidanceregulation/fsma/ucm334115.htm. [Google Scholar]
- 6.Kataoka A, Enache E, Black DG, Elliott PH, Napier CD, Podolak R, Hayman MM. 2014. Survival of Salmonella Tennessee, Salmonella Typhimurium DT104, and Enterococcus faecium in peanut paste formulations at two different levels of water activity and fat. J Food Prot 77:1252–1259. doi: 10.4315/0362-028X.JFP-13-553. [DOI] [PubMed] [Google Scholar]
- 7.Burnett S, Gehm E, Weissinger W, Beuchat L. 2000. Survival of Salmonella in peanut butter and peanut butter spread. J Appl Microbiol 89:472–477. doi: 10.1046/j.1365-2672.2000.01138.x. [DOI] [PubMed] [Google Scholar]
- 8.Enache E, Kataoka A, Hayman M, Podolak R, Black DG, Elliott PH, Whiting R. 2013. Heat resistance of Salmonella Tennessee in peanut paste formulations at four different levels of fat and water activity, poster P2-06. Int Assoc Food Prot Ann Meet, Charlotte, NC, 28 to 31 July 2013. [Google Scholar]
- 9.Finn S, Condell O, McClure P, Amézquita A, Fanning S. 2013. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front Microbiol 4:331. doi: 10.3389/fmicb.2013.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng X, Li Z, Zhang W. 2012. Transcriptome sequencing of Salmonella enterica serovar Enteritidis under desiccation and starvation stress in peanut oil. Food Microbiol 30:311–315. doi: 10.1016/j.fm.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Bhaskara A, Megalis C, Tortorello ML. 2012. Transcriptomic analysis of Salmonella desiccation resistance. Foodborne Pathog Dis 9:1143–1151. doi: 10.1089/fpd.2012.1254. [DOI] [PubMed] [Google Scholar]
- 12.Syamaladevi RM, Tadapaneni RK, Xu J, Villa-Rojas R, Tang J, Carter B, Sablani S, Marks B. 2016. Water activity change at elevated temperatures and thermal resistance of Salmonella in all purpose wheat flour and peanut butter. Food Res Int 81:163–170. doi: 10.1016/j.foodres.2016.01.008. [DOI] [Google Scholar]
- 13.Syamaladevi RM, Tang J, Villa-Rojas R, Sablani S, Carter B, Campbell G. 2016. Influence of water activity on thermal resistance of microorganisms in low-moisture foods: a review. Compr Rev Food Sci Food Safety 15:353–370. doi: 10.1111/1541-4337.12190. [DOI] [PubMed] [Google Scholar]
- 14.Labuza T, Kaanane A, Chen J. 1985. Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci 50:385–392. doi: 10.1111/j.1365-2621.1985.tb13409.x. [DOI] [Google Scholar]
- 15.Labuza TP, Altunakar L. 2007. Water activity prediction and moisture sorption isotherms, p 109–154. In Barbosa-Cánovas GV, Fontana AJ Jr, Schmidt SJ, Labuza TP (ed), Water activity in foods: fundamentals and applications, 1st ed Blackwell Publishing, Ames, IA. [Google Scholar]
- 16.Bandyopadhyay S, Weisser H, Loncin M. 1980. Water adsorption isotherms of foods at high temperatures. Lebensm Wissensch Technol 13:182–185. [Google Scholar]
- 17.Quirijns EJ, Van Boxtel AJ, van Loon WK, Van Straten G. 2005. Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agric 85:1805–1814. doi: 10.1002/jsfa.2140. [DOI] [Google Scholar]
- 18.Kaymak-Ertekin F, Gedik A. 2004. Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. LWT Food Sci Technol 37:429–438. doi: 10.1016/j.lwt.2003.10.012. [DOI] [Google Scholar]
- 19.Almond Board of California. 2014. Guidelines for using Enterococcus faecium NRRL B-2354 as a surrogate microorganism in almond process validation. Guideline 7/14. Almond Board of California, Modesto, CA. [Google Scholar]
- 20.Jeong S, Marks BP, Ryser ET. 2011. Quantifying the performance of Pediococcus sp. (NRRL B-2354: Enterococcus faecium) as a nonpathogenic surrogate for Salmonella Enteritidis PT30 during moist-air convection heating of almonds. J Food Prot 74:603–609. doi: 10.4315/0362-028X.JFP-10-416. [DOI] [PubMed] [Google Scholar]
- 21.Ceylan E, Bautista DA. 2015. Evaluating Pediococcus acidilactici and Enterococcus faecium NRRL B-2354 as thermal surrogate microorganisms for Salmonella for in-plant validation studies of low-moisture pet food products. J Food Prot 78:934–939. doi: 10.4315/0362-028X.JFP-14-271. [DOI] [PubMed] [Google Scholar]
- 22.Bianchini A, Stratton J, Weier S, Hartter T, Plattner B, Rokey G, Hertzel G, Gompa L, Martinez B, Eskridge AM. 2012. Validation of extrusion as a killing step for Enterococcus faecium in a balanced carbohydrate-protein meal by using a response surface design. J Food Prot 75:1646–1653. doi: 10.4315/0362-028X.JFP-12-085. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Kornacki JL, Zhang G, Lin C-M, Doyle MP. 2007. Development of thermal surrogate microorganisms in ground beef for in-plant critical control point validation studies. J Food Prot 70:952–957. doi: 10.4315/0362-028X-70.4.952. [DOI] [PubMed] [Google Scholar]
- 24.Labuza TP. 1975. Sorption phenomena in foods: theoretical and practical aspects, p 197–219. In Rha C. (ed), Theory, determination and control of physical properties of food materials. Springer, Dordrecht, Netherlands. [Google Scholar]
- 25.Tadapaneni RK, Yang R, Carter B, Tang J. 2017. A new method to determine the water activity and the net isosteric heats of sorption for low moisture foods at elevated temperatures. Food Res Int 102:203–212. doi: 10.1016/j.foodres.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 26.Smith DF, Hildebrandt IM, Casulli KE, Dolan KD, Marks BP. 2016. Modeling the effect of temperature and water activity on the thermal resistance of Salmonella Enteritidis PT 30 in wheat flour. J Food Prot 79:2058–2065. doi: 10.4315/0362-028X.JFP-16-155. [DOI] [PubMed] [Google Scholar]
- 27.Villa-Rojas R, Tang J, Wang S, Gao M, Kang D-H, Mah J-H, Gray P, Sosa-Morales ME, López-Malo A. 2013. Thermal inactivation of Salmonella Enteritidis PT 30 in almond kernels as influenced by water activity. J Food Prot 76:26–32. doi: 10.4315/0362-028X.JFP-11-509. [DOI] [PubMed] [Google Scholar]
- 28.Tadapaneni RK, Syamaladevi RM, Villa-Rojas R, Tang J. 2017. Design of a novel test cell to study the influence of water activity on the thermal resistance of Salmonella in low-moisture foods. J Food Eng 208:48–56. doi: 10.1016/j.jfoodeng.2017.03.025. [DOI] [Google Scholar]
- 29.Wahlgren M, Arnebrant T, Lundström I. 1995. The adsorption of lysozyme to hydrophilic silicon oxide surfaces: comparison between experimental data and models for adsorption kinetics. J Colloid Interf Sci 175:506–514. doi: 10.1006/jcis.1995.1482. [DOI] [Google Scholar]
- 30.Gomez-Sjoberg R, Morisette DT, Bashir R. 2005. Impedance microbiology-on-a-chip: microfluidic bioprocessor for rapid detection of bacterial metabolism. J Microelectromech Syst 14:829–838. doi: 10.1109/JMEMS.2005.845444. [DOI] [Google Scholar]
- 31.Syamaladevi RM, Tang J, Zhong Q. 2016. Water diffusion from a bacterial cell in low-moisture foods. J Food Sci 81:R2129–R2134. doi: 10.1111/1750-3841.13412. [DOI] [PubMed] [Google Scholar]
- 32.Leistner L, Rodel W. 1976. Stability of intermediate moisture foods with respect to microorganisms. Intermed Moisture Foods 1976:120–137. [Google Scholar]
- 33.Bianchini A, Stratton J, Weier S, Hartter T, Plattner B, Rokey G, Hertzel G, Gompa L, Martinez B, Eskridge KM. 2014. Use of Enterococcus faecium as a surrogate for Salmonella enterica during extrusion of a balanced carbohydrate-protein meal. J Food Prot 77:75–82. doi: 10.4315/0362-028X.JFP-13-220. [DOI] [PubMed] [Google Scholar]
- 34.Villa-Rojas R, Zhu M-J, Marks BP, Tang J. 2017. Radiofrequency inactivation of Salmonella Enteritidis PT 30 and Enterococcus faecium in wheat flour at different water activities. Biosyst Eng 156:7–16. doi: 10.1016/j.biosystemseng.2017.01.001. [DOI] [Google Scholar]
- 35.Reference deleted.
- 36.Reference deleted.
- 37.Leuenberger P, Ganscha S, Kahraman A, Cappelletti V, Boersema PJ, von Mering C, Claassen M, Picotti P. 2017. Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. Science 355:eaai7825. doi: 10.1126/science.aai7825. [DOI] [PubMed] [Google Scholar]
- 38.Sunde EP, Setlow P, Hederstedt L, Halle B. 2009. The physical state of water in bacterial spores. Proc Natl Acad Sci U S A 106:19334–19339. doi: 10.1073/pnas.0908712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad N, Tsai H-C, Hildebrandt I, Zhu M-J, Tang J, Ryser E, Marks B. 2017. Validation of Enterococcus faecium as a Salmonella surrogate in thermal treatment of almond meal, poster P3-02. Int Assoc Food Prot Ann Meet, Tampa, FL, 9 to 12 July 2017. [Google Scholar]
- 40.Gaillard S, Leguérinel I, Mafart P. 1998. Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores. J Food Sci 63:887–889. doi: 10.1111/j.1365-2621.1998.tb17920.x. [DOI] [Google Scholar]
- 41.Greenspan L. 1977. Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bureau Stand 81:89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- 42.Hildebrandt IM, Marks BP, Ryser ET, Villa-Rojas R, Tang J, Garces-Vega FJ, Buchholz SE. 2016. Effects of inoculation procedures on variability and repeatability of Salmonella thermal resistance in wheat flour. J Food Prot 79:1833–1839. doi: 10.4315/0362-028X.JFP-16-057. [DOI] [PubMed] [Google Scholar]
- 43.Gibbard HF Jr, Scatchard G. 1973. Liquid-vapor equilibrium of aqueous lithium chloride, from 25° to 100°C and from 1.0 to 18.5 molal, and related properties. J Chem Eng Data 18:293–298. doi: 10.1021/je60058a011. [DOI] [Google Scholar]
- 44.Harris LJ, Uesugi AR, Abd SJ, McCarthy KL. 2012. Survival of Salmonella Enteritidis PT 30 on inoculated almond kernels in hot water treatments. Food Res Int 45:1093–1098. doi: 10.1016/j.foodres.2011.03.048. [DOI] [Google Scholar]
- 45.Liu S, Zhong Q, Smith D, Villa-Rojas R, Tang J, Zhu M-J, Marks B. 2015. Validation of Enterococcus faecium NRRL B2354 as a surrogate for Salmonella in thermal treatment of wheat flour at different water activities, poster P1-63. Int Assoc Food Prot Ann Meet, Portland, OR, 25 to 28 July 2015. [Google Scholar]
- 46.Peleg M. 2006. Advanced quantitative microbiology for foods and biosystems: models for predicting growth and inactivation. CRC Press, Boca Raton, FL. [Google Scholar]
- 47.Draper NR, Smith H, Pownell E. 1966. Applied regression analysis, vol 3 Wiley, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.