Fig. 1. The multiple steps of preprotein import into chloroplasts.
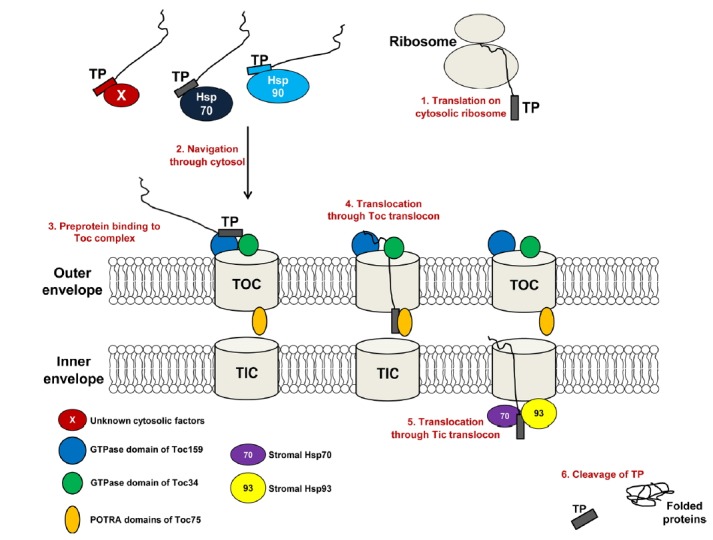
The transit peptide (TP) is a signal sequence in chloroplast interior proteins. In the cytosol, the TP is recognized by cytosolic chaperones such as Hsp70, Hsp90, or factors yet to be identified, which leads to the targeting of preproteins to the chloroplast. At the outer envelope membrane of the chloroplast, the TP interacts with the GTPase receptors Toc159 and Toc34 of the Toc complex, which initiates the translocation of the preprotein through the import channel at the outer envelope membrane. The TP of the preprotein is released from the Toc complex and recognized by the POTRA domains of the Toc75 channel, which coordinate the transfer of the preprotein to the Tic complex. The TP is released from the Tic complex and interacts with stromal chaperone Hsp70 or Hsp93, which has ATPase activity, allowing them to pull the preprotein into the chloroplast. The TP is cleaved off by a stromal processing peptidase during or after the import process.
