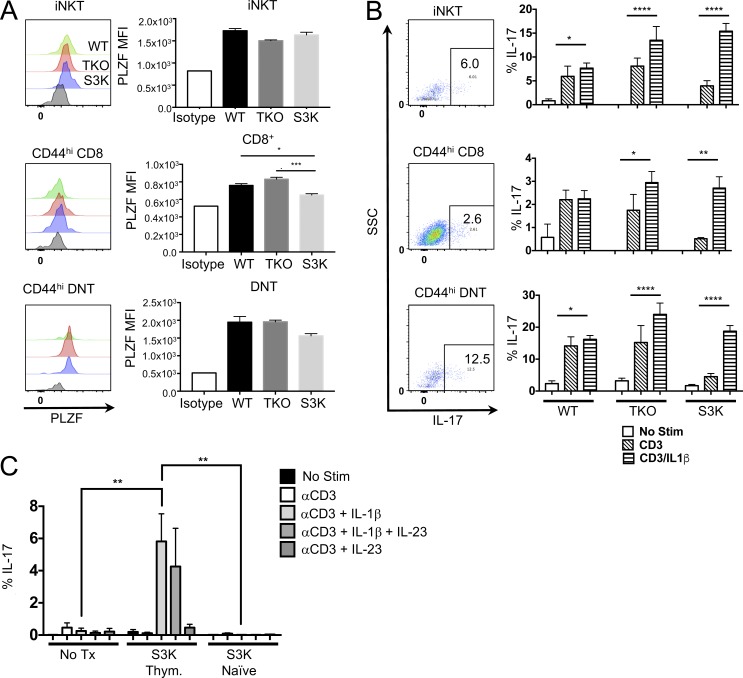
Figure 4.
STAT-3–independent IL-17–producing innate-like T cells exist in the thymus. (A and B) Single-cell suspensions of thymuses from 6-wk-old WT, TKO, or STAT-3 KO (S3K) mice were either stained for PLZF expression (A) or stimulated with CD3 and IL-1β for 72 h (B). Gating strategies are depicted in Fig. S2 A. Histograms represent the PLZF staining. Flow plots represent staining pattern in the thymus after stimulation with CD3 plus IL-1β. Bars in graphs represent the MFI of PLZF staining ± SEM (n = 3 for WT and TKO and n = 5 for S3K; *, P = 0.0130; ***, P = 0.0006). Data represent two independent experiments. (B) Flow plots represent the staining pattern after stimulation with either CD3 or CD3 and IL-1β (no stim samples yielded very few cells). Brefeldin A was added the last 6 h of culture, and IL-17 was assessed by flow cytometry. Bars represent the mean frequency ± SEM of cells that were IL-17+ (n = 3 for WT and TKO and n = 5 for S3K; *, P < 0.05; **, P < 0.01; ****, P < 0.0001). Statistical significance was determined using ANOVA. (C) 2 × 104 CD44hi CD8/DNT S3K thymocytes or 2 × 105 naive CD44hi CD8/DNT S3K cells were transferred i.v. into Rag2/Il2rg DKO mice. After 4 wk, splenic cells were isolated and stimulated with CD3 and IL-1β and/or IL-23 for 72 h. Brefeldin A was added the last 6 h of culture, and cells were stained for intracellular IL-17. Bars represent the mean frequency ± SEM of IL-17+ cells after culture (n = 3 for WT and S3K naive, n = 5 for S3K Thym.). Data represent two independent experiments. Statistical significance was determine by ANOVA (**, P = 0.002).