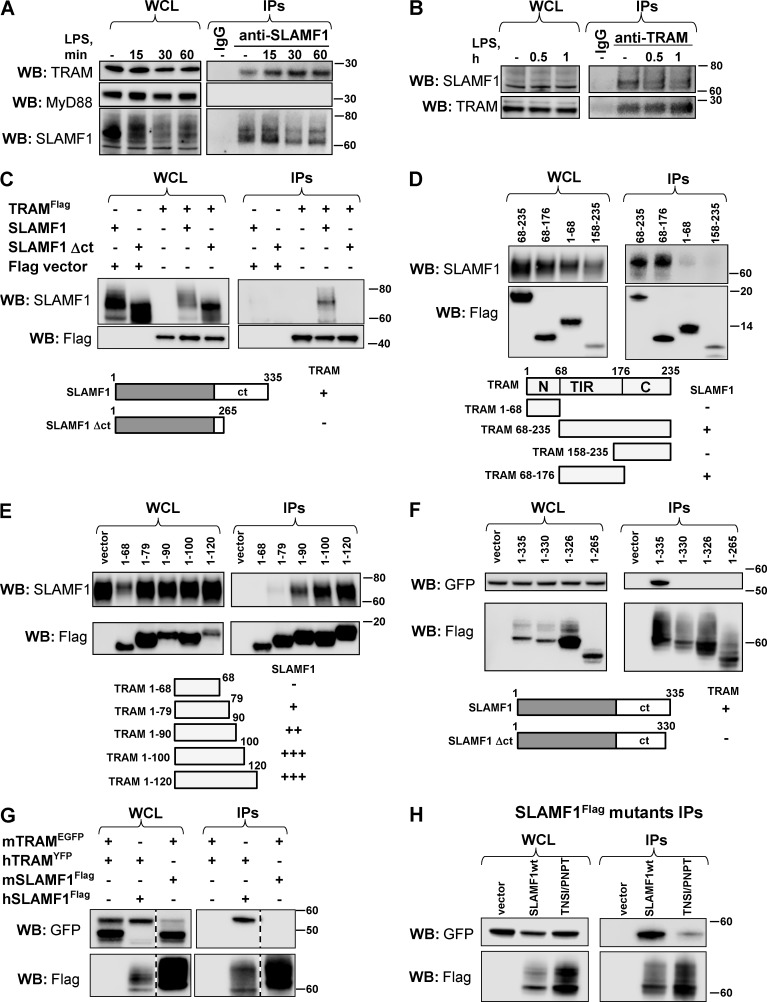
Figure 6.
SLAMF1 interacts with TRAM protein. (A) Endogenous IPs using specific anti-SLAMF1 mAbs from macrophages stimulated by LPS. (B) Endogenous IPs using anti-TRAM polyclonal antibodies from macrophages stimulated by LPS. (C) TRAMFlag-precipitated SLAMF1 and SLAMF1ct was needed for interaction with TRAM. (D) Coprecipitation of TRAM deletion mutants: TIR domain (68–235), short TRAM TIR domain (68–176 aa), and N-terminal (1–68 aa) or C-terminal (158–235 aa) domains with SLAMF1 protein. (E) Coprecipitation of TRAM deletion mutants containing the N-terminal part of TRAM TIR domain with SLAMF1. (F) Coprecipitation of SLAMF1Flag deletion mutants with TRAMYFP. (G) Coprecipitation of human SLAMF1Flag with human TRAMYFP and of mouse SLAMF1Flag with mouse TRAMEGFP. Black dashed lines indicate that intervening lanes have been spliced out. (H) Human SLAMF1 cytoplasmic tail coprecipitation with TRAMYFP with or without amino acid substitutions at 321–324. Graphs under C–F summarize the IPs’ results. Indicated constructs were transfected to HEK293T cells, and anti-Flag agarose was used for the IPs. For endogenous IPs, specific SLAMF1 or TRAM antibodies were covalently coupled to beads. At least three independent experiments were carried out for anti-Flag IPs, and five independent experiments were carried out for the endogenous IPs, and one representative experiment is shown for each. Molecular weight is given in kilodaltons. WB, Western blot; WCL, whole-cell lysate.