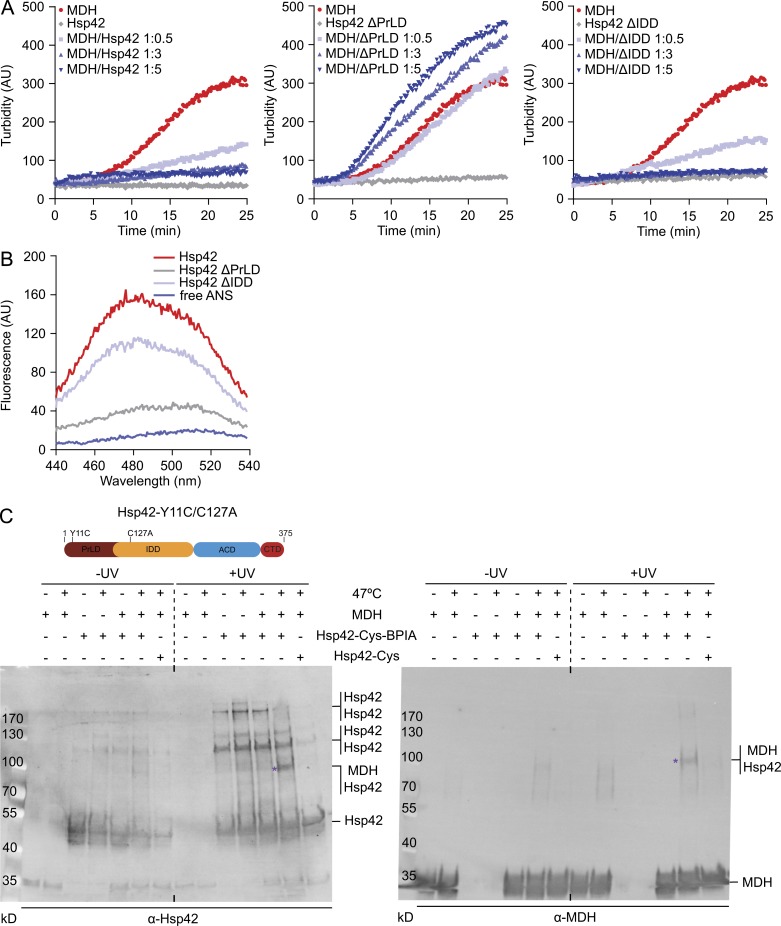
Figure 5.
PrLD is the major Hsp42 substrate interaction site. (A) MDH was denatured for 30 min at 47°C in the absence or presence of Hsp42 WT and deletion mutants at indicated ratios. All control Hsp42 proteins were heated alone. The formation of MDH aggregates was followed by turbidity measurements. (B) Overall surface hydrophobicity of Hsp42 WT and deletion mutants probed by ANS fluorescence. Fluorescence of free ANS is provided as reference. (C) Cross-linking of BPIA-labeled Hsp42-Y11C/C127A (Hsp42-Cys) to unfolded MDH. MDH and Hsp42 alone or MDH/Hsp42-Cys (unlabeled and BPIA-labeled) mixtures (1:5 ratio) were incubated at 30°C or 47°C. Samples were afterward exposed to UV (+/− UV), and cross-link products were analyzed by immunoblot analysis using MDH and Hsp42-specific antibodies. Asterisks indicate Hsp42-MDH cross-link products. A schematic diagram of Hsp42-Y11C/C127A is shown at top.