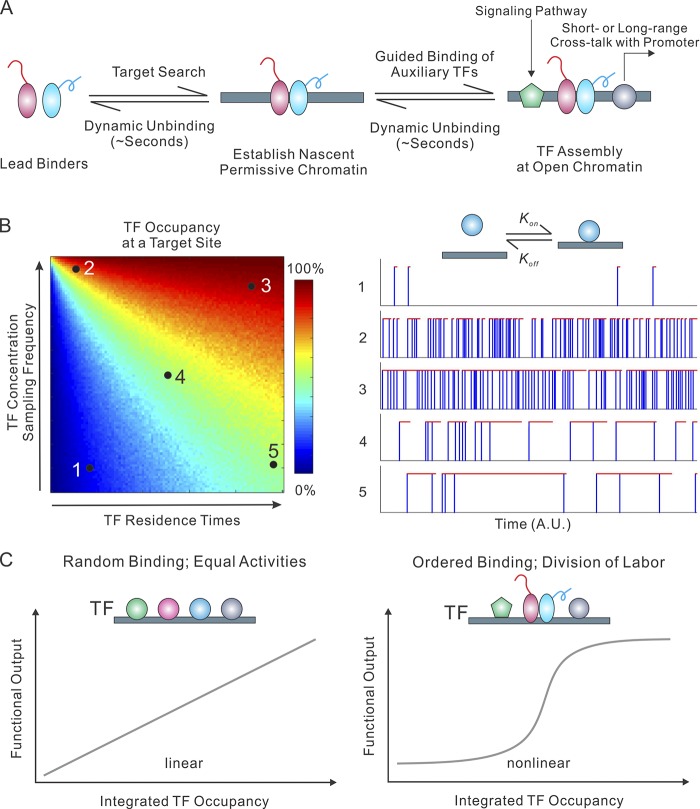
Figure 2.
TF binding dynamics at enhancers. (A) TF binding kinetics at cis-regulatory elements is dynamic in live cells. Accumulating evidence suggests that a small group of site-specific TFs act as lead or pioneer binders that efficiently scan and engage with silent chromatin, establishing a permissive chromatin for the subsequent binding of auxiliary TFs, which in turn reinforces an open-chromatin state. TFs bound within the assembly mediate distinct functions (e.g., signal transduction and interplay with core promoter), suggesting a functional division of labor for TFs. (B) The TF temporal occupancy pattern at a specific target site is exquisitely regulated by TF concentration in the nucleus and TF residence times at the target site (left). For this numerical simulation, random TF residence times (R; AU) and sampling intervals (I; AU) were generated based on a log-normal distribution. We set the σ to 1/4 Log(R) or Log(I). The selection of this distribution is based on the observation that the log-scale value of Sox2 residence times on specific DNA probes roughly follows a normal distribution (Chen et al., 2014b). For each pair of mean TF residence times (R) and sampling intervals (I), 1,000 continuous binding events were simulated, and the TF occupancy (color-encoded) of the target site was calculated based on the total binding on/off durations. Representative binding traces of a TF at the target site with defined parameters (1–5) are shown on the right. Blue vertical lines represent TF sampling events. Red horizontal lines reflect TF dwelling events at the target site. (C) The relationship between TF binding dynamics and the functional output of an enhancer. If multiple TFs have an equal ability to bind to the target site and activate gene expression, the functional output of an enhancer should be linearly proportional to integrated TF occupancy at the enhancer. An ordered TF assembly and functional division-of-labor mechanism could potentially generate rapid and nonlinear functional outputs.