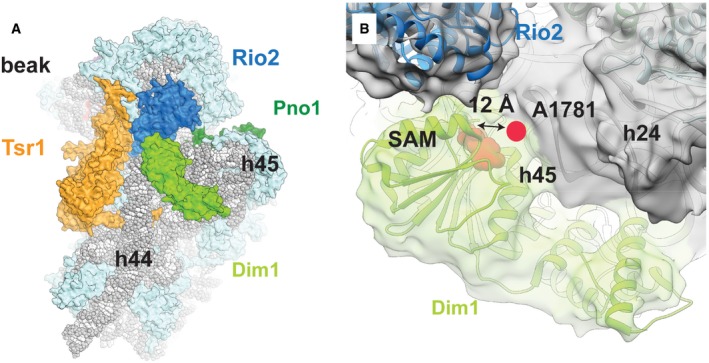
Dim1 (bright green) binds not only to helix 44, but also helices 45 and 24. Rigid‐body fitting of the available crystal structure of human Dim1 in complex with S‐adenosyl methionine (SAM) (pink) (PDB: 1ZQ9) into the cryo‐EM density shows that the previously described active site of Dim1 would be located too distant from its methylation target, A1781 (pink circle), to allow the methylation reaction to take place. Additionally, the unmethylated helix 45 has been reported to be rotated upward to allow access for Dim1 (Johnson
et al,
2017). Thus, Dim1 has likely already methylated A1781 and A1782, as shown previously by primer extension analysis of the Nob1‐D15N particle (Lebaron
et al,
2012).