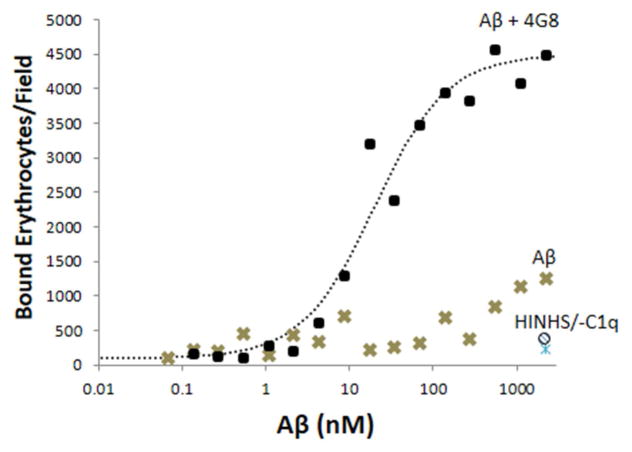
Fig. 3. Erythrocyte capture of Aβ and Aβ ICs.
In erythrocyte adhesion assays, erythrocytes bound to Aβ-coated and Aβ/4G8 IC-coated plates in a dose-dependent manner (P < 0.001) and binding was significantly greater for Aβ ICs (P < 0.001). Abolition of effects by heat-inactivated NHS at the highest dose of Aβ ICs and Aβ alone (HINHS and ○ symbol at bottom right of graph) demonstrated specificity to complement reactions as opposed to erythrocyte binding by mechanisms other than complement. Abolition of effects by C1q-depleted serum (-C1q and △ symbol at bottom right of graph) confirmed this finding and, in addition, strongly suggested that erythrocyte capture of Aβ is primarily mediated by the classical, rather than the alternative complement pathway. Each data point represents the mean of duplicates. The vast majority of data points exhibited standard errors that were too small to show clearly. For the Aβ alone condition, the average standard error of the mean was 111.3 bound erythrocytes/field (range of 7.8 to 422.5). For the Aβ IC condition, the average standard error of the mean was 321.0 bound erythrocytes/field (range of 9.9 to 996.7).