Figure 1. Efficacy End Points.
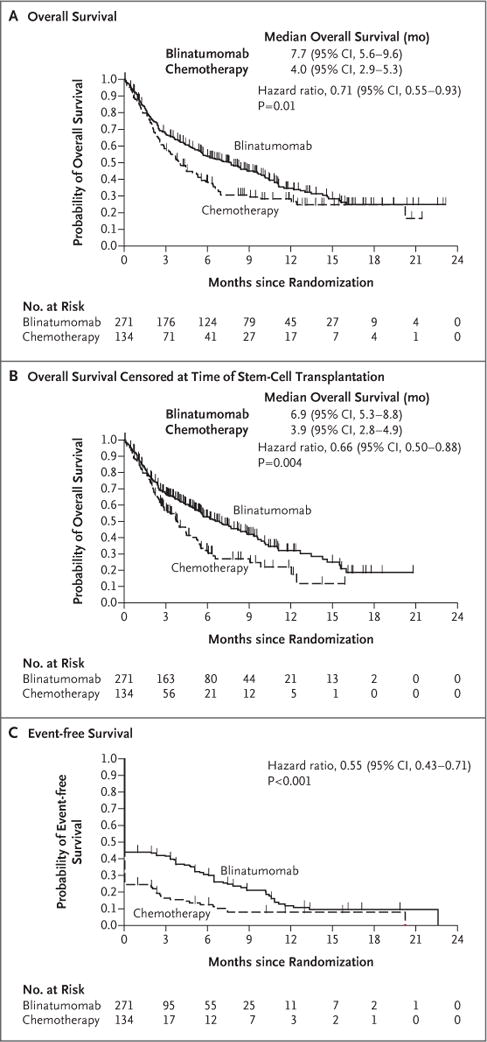
Panel A shows the probability of overall survival in the two groups. Overall survival was calculated as the time from randomization to death from any cause. The median duration of follow-up for overall survival was 11.7 months in the blinatumomab group and 11.8 months in the chemotherapy group. Panel B shows the probability of overall survival in the two groups (also calculated as the time from randomization to death from any cause) when data were censored at the time of allogeneic stem-cell transplantation. The median duration of follow-up for this analysis of overall survival was 7.0 months in the blinatumomab group and 6.0 months in the chemotherapy group. Panel C shows the probability of event-free survival, which was calculated as the time from randomization until relapse after complete remission with full, partial, or incomplete hematologic recovery, or death; patients who did not achieve a complete re-mission with full, partial, or incomplete hematologic recovery were assigned an event-free duration of 1 day. The median duration of follow-up for event-free survival was 7.8 months in the blinatumomab group and 10.2 months in the chemotherapy group. All three analyses were performed in the intention-to-treat population. P values were determined by means of stratified log-rank tests.
