Figure 4. TgASP3 mutants are functional and not detrimental to parasite fitness.
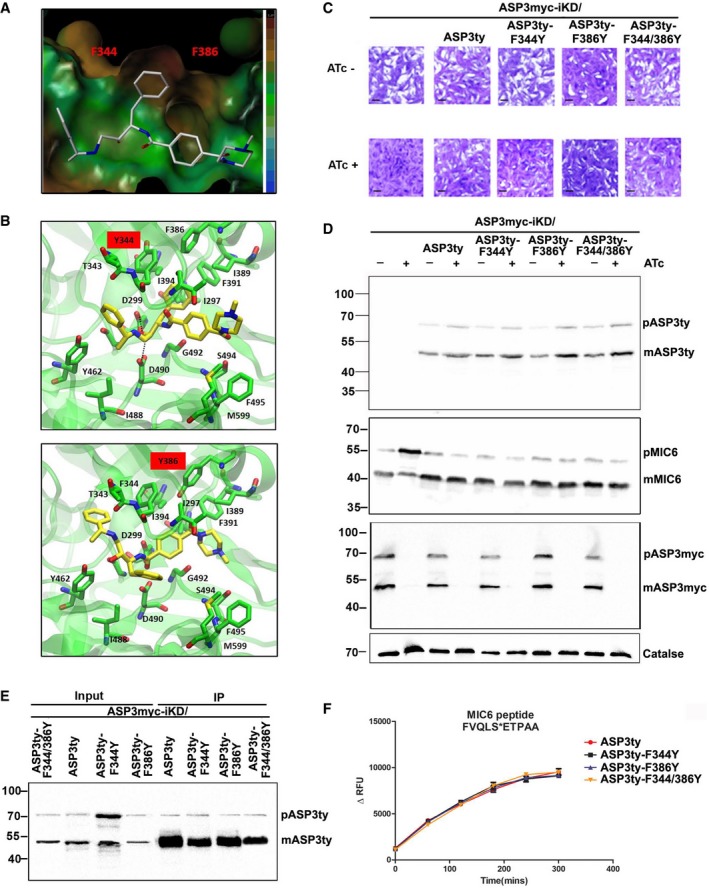
- Lipophilic potential surface of the binding site of TgASP3 with compound 49c. The colored legend on the right shows the increasing of the lipophilicity from the blue color (bottom) to the brown (upper), highlighting the presence of a hydrophobic cavity between the two F344 and F386.
- Molecular docking predictions of the possible effects in the binding mode of 49c in the presence of the two mutants ASP3‐F344Y (upper panel) and ASP3‐F386Y (lower panel)
- Plaque assay was performed in parental stain ASP3myc‐iKD or parental strain complemented with wild‐type ASP3 (ASP3ty) or with mutant forms of ASP3 (ASP3ty‐F344Y, ASP3ty‐F386Y, ASP3ty‐F344Y/F386Y) in the UPRT locus. Knockdown of ASP3 in presence of ATc resulted in complete impairment of the lytic cycle, as assessed by plaque formation after 7 days, in parental ASP3myc‐iKD strain. Complementation with ASP3ty or with ASP3 mutants (ASP3ty‐F344Y, ASP3ty‐F386Y, and ASP3ty‐F344Y/F386Y) fully restored plaque formation. Scale bar represents 1 μm.
- Western blots analysis comparing lysate of parental ASP3myc‐iKD strain and complemented strain (ASP3myc‐iKD/ASP3ty, ASP3myc‐iKD/ASP3ty‐F344Y, ASP3myc‐iKD/ASP3ty‐F386Y, ASP3myc‐iKD/ASP3ty‐F344Y/F386Y) ± ATc for 48 h. Significant accumulation of TgMIC6 precursor form with reduction of mature form was observed in iKDASP3myc parasites by ASP3 depletion. Parasites complemented with either wild type or with the mutant form of ASP3 as well as untreated parasite showed proper processing of TgMIC6. Regulation of myc‐tagged inducible copy of ASP3 was shown by probing with α‐myc antibody. Catalase is used as loading control.
- Lysate of wild‐type ASP3ty and mutant form of ASP3 (ASP3ty‐F344Y, ASP3ty‐F386Y, ASP3ty‐F344Y/F386Y) stably expressed in the UPRT locus of ASP3myc‐iKD parasites was used to immunoprecipitate (IP) wild‐type or mutant forms of ASP3 using anti‐ty couple beads. Input and bound fractions were analyzed by Western blot and revealed the presence of precursor (pASP3ty) and mature form (mASP3ty) of ASP3ty.
- Immunoprecipitated wild‐type ASP3ty and its mutant forms (ASP3ty‐F344Y, ASP3ty‐F386Y, ASP3ty‐F344Y/F386Y) cleave TgMIC6 fluorogenic peptide (DABCYL‐G‐ FVQLS|ETPAA ‐G‐EDANS) with equal efficiency. Result represents mean ± SD, n = 3, of three independent experiments.
Source data are available online for this figure.
