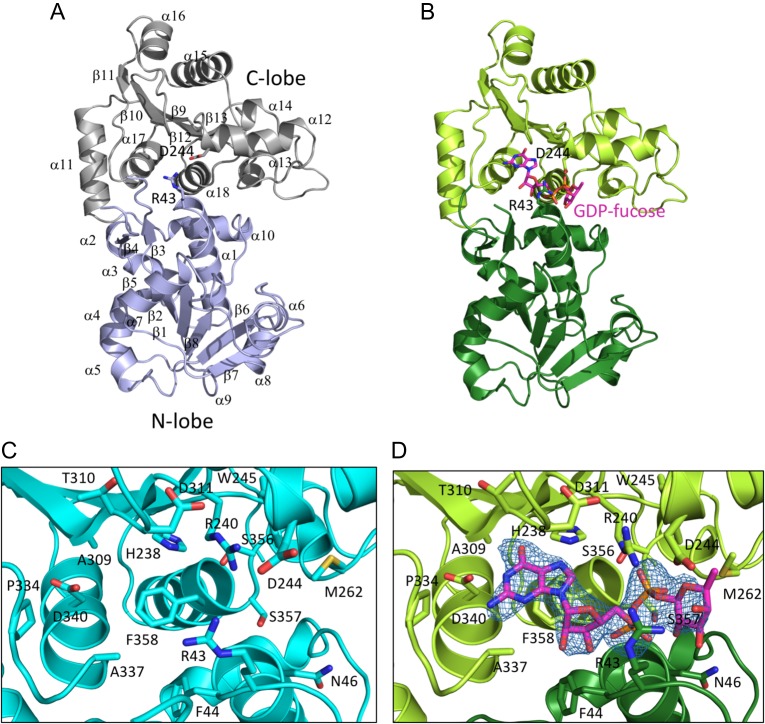
Fig. 2.
Structure overview. (A) Ribbon diagram of the unliganded enzyme, showing overall architecture of the enzyme. The N-terminal lobe is colored slate blue, and the C-terminal lobe is gray. Secondary structure elements are indicated; R43 and D244 are shown as sticks. (B) Ribbon diagram of the enzyme complexed with GDP-fucose. The N-terminal lobe is dark green, the C-terminal lobe is light green, and the GDP-fucose is in CPK colors, with carbon atoms colored magenta. R43 and D244 are shown as sticks. (C) Zoomed in view of the active site of the unliganded enzyme. Side chains of residues in the active site are in CPK colors, with carbon atoms in cyan. (D) Zoomed in view of the active site of the enzyme with GDP-fucose bound. Side chains of residues in the active site are rendered as sticks in CPK colors, with carbon atoms in green. The bound GDP-fucose is rendered as sticks in CPK colors, with carbon atoms in magenta. An Fo–Fc difference map for the GDP-fucose ligand is shown in blue mesh, contoured at 3σ. This figure is available in black and white in print and in color at Glycobiology online.