Abstract
Background and Aims
Exclusive enteral nutrition [EEN] is recommended as a first-line induction therapy for paediatric Crohn’s disease [CD] although corticosteroids [CS] are still used commonly. Our aim was to compare short- and long-term disease outcomes of paediatric CD patients initially managed with either EEN or CS.
Methods
Medical records of newly diagnosed paediatric CD patients treated with EEN or CS as induction therapy were retrospectively reviewed. To minimise selection bias inherent in observational cohort studies, propensity analysis was carried out. Data on anthropometrics, medical history, and presenting phenotype were collected at time of diagnosis [baseline]; outcomes of interest, including medication use, hospitalisation, surgical procedures, and disease progression were assessed up to 6 years following diagnosis.
Results
Of 127 patients reviewed, a total of 111 propensity-score matched CD patients receiving EEN [n = 76] or CS [n = 35] were analysed. By 4–12 weeks of induction therapy, 86.6% of EEN-treated patients achieved remission (Paediatric Crohn’s Disease Activity Index [PCDAI] ≤ 7.5) compared with 58.1% of patients in the CS-treated group [p < 0.01]. Choice of EEN over CS for induction was associated with avoidance of corticosteroids over a 6-year follow-up period. Analysis of long-term linear growth, hospitalisation, need for biologic therapy, or surgical intervention did not reveal any significant differences.
Conclusions
These findings suggest that EEN induction therapy is more effective in achieving early remission and is associated with long-term steroid avoidance without increased use of biologics or need for surgery.
Keywords: EEN, corticosteroids, propensity score
1. Introduction
Up to 25% of Crohn’s disease [CD] patients are diagnosed during childhood, at which time disease is characterised by more extensive intestinal involvement compared with adult-onset CD, with a comparable progression toward complicated disease.1–3 Paediatric-onset patients are also at risk for faltering growth, which is commonly present at diagnosis.4 Short-term treatment decisions to achieve remission in the paediatric population must therefore take into account long-term outcomes including modification of disease progression, prevention of adverse effects of treatment, and guarantee of suitable growth and development.
Induction of remission in paediatric patients with active CD can be achieved by exclusive enteral nutrition [EEN] or corticosteroids [CS]. While meta-analyses of adult studies suggest superiority of CS, paediatric studies have shown that EEN is at least as effective as CS for inducing remission, and is more effective than CS in improving nutritional status and growth recovery without adverse side effects.5–8 EEN is also capable of achieving mucosal healing, an important therapeutic endpoint that, when achieved early, is associated with fewer hospitalisations, reduced surgical resections and risk of fistulising disease, and less use of biologic (anti-tumour necrosis factor [TNF]) drugs.6,8-12
Despite the reported benefits, EEN is not universally used in paediatric centres. Wide differences have been noted in the use of EEN between gastroenterologists in Europe and North America.13,14 We have recently reported that various factors, including concerns about compliance and costs, may affect the attitudes of health professionals towards EEN and impede its wider use.15 Studies comparing the long-term outcomes of EEN versus CS treatment are limited. Few randomised controlled trials have been performed, and existing observational cohort studies are potentially confounded by differences in patient and/or disease characteristics at diagnosis which may bias treatment choice.
To examine differences in treatment response in our observational cohort, we used propensity-score matching to minimise confounding by indication which results from non-randomised assignment of treatment groups. Propensity-score methodology accounts for selection bias by first matching patients treated with different therapies for the distribution of potential confounders, and then comparing the therapies only within this subsample of matched patients.16,17 Newly-diagnosed children with CD treated with either CS or EEN as induction therapy were evaluated. We compared rates of remission, steroid avoidance, need for anti-TNF therapy, linear growth, and surgical resections for up to 6 years of follow-up.
2. Methods
2.1. Study design and data collection
A single-centre, retrospective analysis was performed on data collected from paediatric CD patients at the IWK Health Centre between January 2001 and March 2015. A prospectively maintained departmental database was used to identify newly diagnosed paediatric CD patients who received either CS or EEN as induction therapy. All study patients had a confirmed CD diagnosis based on established clinical, endoscopic, histological, and/or radiological criteria.18
Information on patient demographics, disease characteristics, medications, and medical and family history were collected by detailed review of medical records. Disease was classified according to the Paris classification system for location [L1: distal 1/3 ileum, limited caecal disease; L2: colonic; L3: terminal ileum and colon; L4a and L4b: oesophagogastric proximal and distal to the ligament of Treitz, respectively] and behaviour [B1: non-stricturing, non-penetrating; B2: stricturing; B3: penetrating].18 For the purposes of analysis, L4a and/or L4b involvement was grouped as L4. Macroscopic involvement was defined by the presence of mucosal ulceration and/or bowel wall thickening on radiography. Macroscopic findings were confirmed by histological features [ie presence of granuloma or cryptitis/crypt abscesses], if available. Perianal disease was defined by the presence of perianal abscesses or fistulae, and did not include isolated presence of skin tags, fissures, or haemorrhoids. Reported history of inflammatory bowel disease [IBD] of any type in a patient’s relative was recorded as ‘positive family history’. Height z-scores [Htz] were calculated using the WHO Anthro Plus v.1.0.4 software.19 We defined Htz of < -1.65 [i.e. below the 5th percentile] as linear growth failure. The Paediatric Crohn’s Disease Activity Index [PCDAI] was used to evaluate disease activity at diagnosis [baseline] and early response to induction therapy [4–12 weeks post-induction].
Short-term outcomes were clinical response to treatment and remission based on PCDAI score. Clinical response was defined as a PCDAI change of ≥ 12.5 points, and remission was defined as a PCDAI ≤ 7.5 points, without the height item.20–22 Long-term outcomes included changes in Htz, subsequent clinical outcomes including hospitalisation directly related to CD, need to commence anti-TNF agent, surgery, and change in disease phenotype. Changes in disease phenotype were assessed at each follow-up; specifically, anatomical extension of disease [confirmed by endoscopy/radiology], progression to stricturing/penetrating disease behaviour, and onset of perianal disease were analysed.
2.2. Sample size calculation
We performed a sample size calculation using 12-week steroid-free rates of remission [PCDAI ≤ 10, or ≤ 7.5 without the height item] reported in the GROWTH CD study comparing EEN-treated and CS-treated paediatric CD patients [73.1% versus 46.3%, respectively].7,23 Considering an approximate 1:2 ratio of CS to EEN patients within our study, we determined that a minimum sample size of 114 patients was required for 80% power to detect this remission outcome [two-sided alpha risk 0.05].
2.3. Propensity-score matching
Induction therapy was selected by the treating physician and/or family, hence patients were not randomised to treatment. Covariates presumed to be associated with the decision of CS or EEN treatment were included in a multinomial logistic regression analysis. The covariates selected were gender, age, weight, height, PCDAI, disease location and behaviour, and presence of perianal disease. Propensity scores [PS] were generated automatically using SPSS [version 20.0, SPSS, Chicago, IL, USA], R 2.12.1 [http://cran.r-project.org], and an R plug-in for SPSS.24
For the matched cohort analysis, patients treated with CS were matched with patients treated with EEN according to the PS, using a 1:2 matching procedure with replacement and a caliper width [ie the allowable standard deviation of PS] of 0.15 resulting in a relatively narrow difference between matched variables, as recommended.25,26 Nearest-neighbour matching with 1:2 replacement was used in order to retain as many CS patients as possible, as there were fewer CS patients eligible for participation in this study.
2.4. Statistical analysis
All statistical calculations were performed using IBM SPSS ver. 20.0 and Graph Pad Prism ver. 5 software. Continuous outcome measures were analysed using Mann-Whitney U test or t test, as appropriate. Categorical data were analysed using chi-square or Fisher’s exact test. T tests were also used to examine differences in Htz, or change in Htz [ΔHtz], between groups at each time point. Repeated measures analysis of variance [ANOVA] was performed to compare Htz between groups over time. Kaplan-Meier survival analysis was performed to evaluate time to first use of anti-TNF therapy.
3. Results
3.1. Baseline characteristics of patients treated with CS versus EEN as induction therapy
Medical charts from 127 children with newly diagnosed CD treated with either EEN [n = 82] or CS [n = 45] as initial therapy were reviewed. Five patients who stopped CS or EEN therapy before 4 weeks of follow-up were excluded. Following propensity-score matching, 111 children with CD treated with either EEN [n = 76] or CS [n = 35] as induction therapy remained in the matched cohort sample. We determined this matched cohort had 78.2% power to detect the remission outcomes reported in the GROWTH CD study [two-sided alpha risk 0.05]. Follow-up of at least 2 years was completed for 109 patients [98.2%]; follow-up data for up to 4 and 6 years were available for 77 [69.4%] and 37 [33.3%] patients, respectively. The median age of diagnosis for patients in this cohort is approximately 12 years, thus the high rate of attrition by 4–6 years is attributed largely to patients transitioning into adult care.
Most CS-treated patients received prednisone [n = 32], with only three patients receiving budesonide. The majority of patients in the EEN group were treated for 8–16 weeks and received formula via nasogastric tube [n = 74]. Two patients elected to ingest formula orally, one of which discontinued after 6 weeks. Baseline clinical and phenotypic characteristics are shown in Table 1. The majority of both CS [62.9%] and EEN [69.7%] patients presented with ileocolonic [L3] disease with or without upper gastrointestinal [GI] [L4] involvement (p = non-significant [NS]). Patient and clinical characteristics were comparable between the CS and EEN groups, with the exception of body mass index [BMI]. BMI z-scores at diagnosis were significantly lower in patients who initiated EEN versus CS [p < 0.05]. Furthermore, 17.1% patients in the EEN group exhibited growth failure compared with 2.9% of patients in the CS group [p = 0.06].
Table 1.
Baseline characteristics of 111 propensity-score matched paediatric CD patients receiving CS or EEN as induction therapy.
| CS | EEN | P value | |
|---|---|---|---|
| n | 35 | 76 | |
| Gender [male/female]a | 9/26 | 28/48 | 0.28 |
| Median age, y [range]a | 12.2 [6.8–16.0] | 11.9 [3.3–16.3] | 0.17 |
| Paris A1a [< 10 y], n [%] | 7 [20] | 12 [15.8] | 0.29 |
| Positive family history, n [%] | 16 [45.7] | 31 [40.7] | 0.63 |
| Disease location, n [%]a | |||
| L1 | 3 [8.6] | 7 [9.2] | 0.58 |
| L2 | 4 [11.4] | 4 [5.3] | |
| L3 | 12 [34.3] | 20 [26.3] | |
| L1+L4 | 1 [2.9] | 4 [5.3] | |
| L2+L4 | 5 [14.3] | 8 [10.5] | |
| L3+L4 | 10 [28.6] | 33 [43.4] | |
| Disease behaviour, n [%]a | |||
| B1 | 35 [100] | 75 [98.7] | 1.00 |
| B2 | 0 | 1 [1.3] | |
| Perianal disease, n [%]a | 4 [11.4] | 3 [3.9] | 0.20 |
| Height z-score [Htz] [mean ± SD]a | -0.01 ± 1.1 | -0.44 ± 1.2 | 0.07 |
| Growth failure [Htz ≤ 1.65] | 1 [2.9] | 13 [17.1] | 0.06 |
| BMI z-score [mean ± SD]a | -0.58 ± 1.3 | -1.2 ± 1.2 | 0.01 |
| Clinical disease severity, PCDAI scorea | |||
| Mild < 30 | 12 [34.3] | 37 [48.7] | 0.27 |
| Moderate ≥ 30 to < 40 | 14 [40.0] | 20 [26.3] | |
| Severe ≥ 40 | 9 [25.7] | 19 [25.0] | |
| Concomitant medicationsb | |||
| Immunomodulators | 14 [40] | 22 [28.9] | 0.25 |
| 5-ASA | 2 [5.7] | 2 [2.6] | 0.59 |
| Antibiotics | 3 [8.6] | 4 [5.3] | 0.68 |
CS, corticosteroid; EEN, exclusive enteral nutrition; y, years; SD, standard deviation; BMI, body mass index; PCDAI, Paediatric Crohn’s Disease Activity Index; 5-ASA, 5-aminosalicylic acid.
aVariables used to compute the propensity scores.
bStarted before Week 4 of induction therapy.
3.2. Clinical response and remission after induction therapy
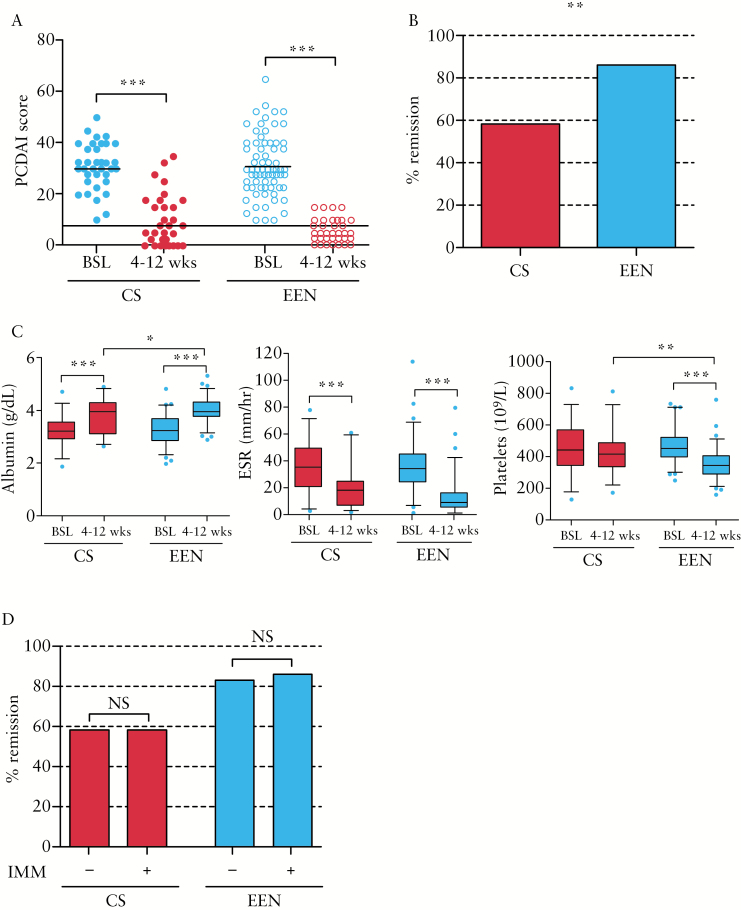
All patients had a PCDAI score ≥ 10 at the time of diagnosis [baseline] before starting induction therapy. The majority of patients in both cohorts had moderate to severe disease activity [PCDAI ≥ 30], with comparable frequencies of severe disease [PCDAI ≥ 40] [Table 1]. More than 80% of patients in both groups exhibited clinical response to treatment [change in PCDAI ≥ 12.5] at 4–12 weeks follow-up [Figure 1A], with 86.6% of EEN-treated patients achieving remission [PCDAI ≤ 7.5] compared with 58.1% in the CS-treated group [p < 0.01] [Table 2 and Figure 1B].
Figure 1.
Clinical response to initial treatment with CS or EEN therapy in newly-diagnosed paediatric CD patients. A. PCDAI scores of CS- or EEN-treated patients at baseline [BSL] and 4–12 weeks post-treatment. B. Percentage of CS- or EEN-treated patients in remission [PCDAI ≤ 7.5] at 4–12 weeks. C. Laboratory values for albumin, ESR, and platelets. D. Percentage of CS- or EEN-treated patients in remission treated with or without concomitant immunomodulator [IMM] within 4 weeks of induction. ***p ≤ 0.001; **p ≤ 0.01; *p < 0.05. CS, corticosteroid; EEN, exclusive enteral nutrition; SD, standard deviation; PCDAI, Paediatric Crohn’s Disease Activity Index; CD, Crohn’s disease; ESR, erythrocyte sedimentation rate.
Table 2.
Comparison of clinical response and remission based on PCDAI in CD patients receiving CS or EEN for induction therapy.
| CS | EEN | p-Value | |
|---|---|---|---|
| PCDAI score [median ± SD] | |||
| Baseline | 30 ± 9.1 | 30 ± 11.6 | 0.43 |
| 4–12 weeks | 7.5 ± 10.2 | 2.5 ± 4.3 | < 0.01 |
| Response by 12 weeks [%] | 80.6 | 89.6 | 0.23 |
| Remission by 12 weeks [%] | 58.1 | 86.6 | < 0.01 |
CS, corticosteroid; EEN, exclusive enteral nutrition; SD, standard deviation; PCDAI, Paediatric Crohn’s Disease Activity Index; CD, Crohn’s disease.
Laboratory values for albumin, erythrocyte sedimentation rate [ESR], and platelets were similar between groups at baseline assessment [Figure 1C]. By 4–12 weeks of therapy, EEN-treated patients had higher albumin levels and lower platelet levels than CS-treated patients. Pairwise analysis of individual changes in laboratory values by 4–12 weeks of therapy showed that patients in the both groups exhibited significant improvements in albumin and ESR levels; EEN-treated patients also exhibited significantly reduced platelet levels.
Immunomodulator use was comparable between groups, with 40% of CS patients and 28.9% of EEN patients starting an immunomodulator within 4 weeks of starting induction therapy [Table 1]. Patients on concomitant immunomodulator therapy exhibited similar rates of remission as did patients not on immunomodulator, within CS- and EEN-treated groups [Figure 1D].
3.3. Biologic use and corticosteroid avoidance
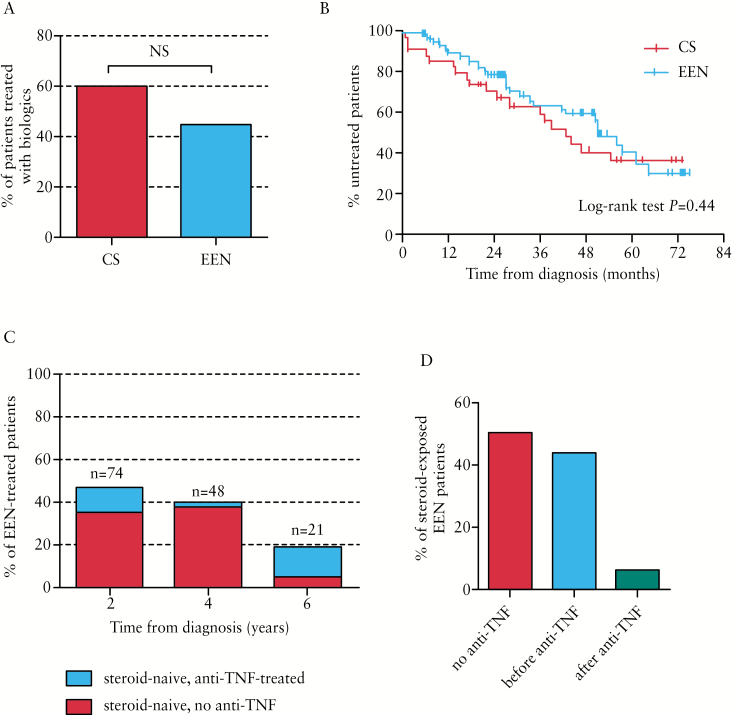
There was no significant difference between groups in the proportion of patients requiring treatment with biologics, with 60% of CS and 44.7% of EEN patients receiving anti-TNF by the maximum follow-up [p = NS] [Figure 2A]. Within the first 2 years of diagnosis, 40% of CS-treated patients received anti-TNF therapy, compared with 24.3% of EEN-treated patients [p = 0.09]. There was no significant difference in the time to first use of anti-TNF therapy between groups [Figure 2B].
Figure 2.
Biologic and steroid use in CS- and EEN-treated CD patients by maximum follow-up. A. Percentage of patients treated with CS or EEN at diagnosis who received anti-TNF therapy by maximum follow-up. B. Kaplan-Meier curve showing time from diagnosis to first use of anti-TNF therapy. C. Percentages of EEN-treated patients who remained steroid-naïve, with or without anti-TNF exposure by 2, 4, and 6 years’ follow-up. D. Percentages of EEN-treated patients exposed to steroids by maximum follow-up who were: not exposed to anti-TNF; or received first exposure to steroids before or after first exposure to anti-TNF. CS, corticosteroid; EEN, exclusive enteral nutrition; CD, Crohn’s disease; TNF, tumour necrosis factor.
The use of EEN as initial therapy decreased the risk of exposure to CS over a 6-year period, which was most pronounced at 2 and 4 years post-diagnosis with 47.3% and 39.6% of EEN patients remaining steroid-naïve, respectively [Figure 2C]. Notably, the majority of EEN-treated patients who had not been exposed to steroids by 2 and 4 years’ follow-up were also naïve to anti-TNF therapy [74.3% and 94.7% at years 2 and 4, respectively] [Figure 2C], For EEN-treated patients who were exposed to steroids by maximum follow-up, 50% were also treated with anti-TNF therapy. In EEN patients exposed to steroids and anti-TNF, 21 out of 24 [87.5%] received their first exposure to steroids before the start of anti-TNF therapy [Figure 2D].
3.4. Linear growth outcomes
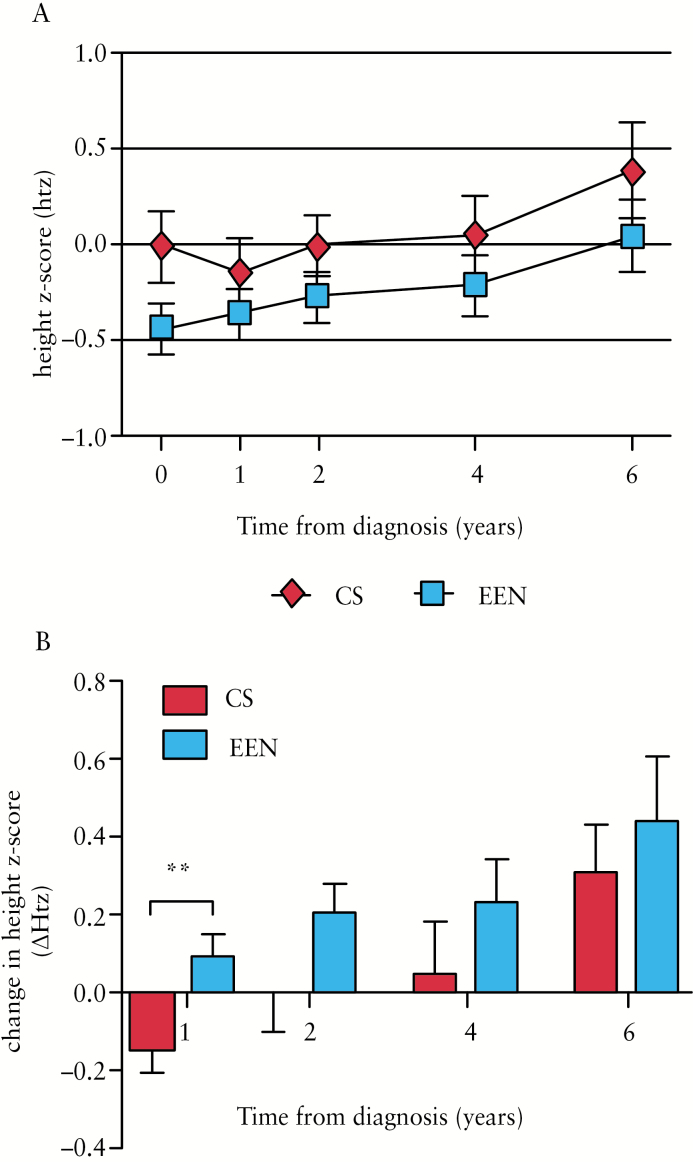
Patients in the EEN-treated group generally exhibited lower height z-scores at baseline [p = 0.07] [Table 1 and Figure 3A], but this difference was no longer observed by 1 year follow-up [p = 0.32], and linear growth remained comparable between CS- and EEN-treated patients at 2, 4, and 6 years’ follow-up [p = 0.24, 0.32, and 0.28, respectively] [Figure 3A]. Repeated measures ANOVA for over-time comparison of Htz between CS- and EEN-treated groups revealed no significant difference over the first 3 years [p = 0.10], or 6 years [p = 0.51] of follow-up. Notably, EEN-treated patients experienced significantly greater linear growth recovery than CS patients 1 year after diagnosis [p < 0.01] [Figure 3B], with an average change in Htz [ΔHtz] of 0.09 versus -0.14 in the CS-treated patients. Indeed, 45/74 [60.8%] EEN-treated patients showed a positive [> 0] change in Htz at the 1 year follow-up compared with only 14/35 [40%] CS patients [p = 0.06].
Figure 3.
Comparison of linear growth outcomes between CS- and EEN-treated CD patients. A. Height z-scores [Htz] of patients treated with CS or EEN at diagnosis and 1,2, 4, and 6 years’ follow-up. B. Changes in height z-scores [ΔHtz] relative to baseline assessment in patients treated with CS or EEN at 1, 2, 4, and 6 years’ follow-up. Data are shown as mean ± standard error of the mean [SEM]. **p < 0.01. CS, corticosteroid; EEN, exclusive enteral nutrition; CD, Crohn’s disease.
3.5. Changes in disease location and behaviour
All patients had a complete endoscopic exploration [upper and lower GI] at the time of diagnosis. Small bowel assessments were performed in 102 of 111 patients, and type of examinations included small bowel follow-through [n = 33], indium scan [n = 77], and magnetic resonance [MR] enterography [n = 8]. In total, 43 of the 111 [38.7%] patients in our cohort already had the maximum disease extent [ie L3+L4] at diagnosis, 10 [28.6%] and 33 [43.4%] of whom belonged to the CS and EEN groups, respectively [Table 1]. Of the remaining 68 patients who could therefore change CD location, only 43 underwent follow-up examination and the majority belonged to the EEN-treated group: 13 [30.2%] CS- versus 30 [69.8%] EEN-treated patients. Altogether, eight patients, all belonging to the EEN-treated group, exhibited anatomical extension of disease by maximum follow-up [Table 3]. Three of the eight patients who exhibited disease extension were treated with immunomodulator during the period in which they received EEN. In the majority of cases, extension was due to increased ileal involvement [seven patients L2 ➔ L3 versus one patient L1 ➔ L3], with one patient also developing proximal small bowel [L4] disease. Of note, one patient found to have ileal disease on follow-up did not have radiological assessment of the small bowel performed at diagnosis.
Table 3.
Comparison of long-term disease progression and complications at maximum follow-up between CS- and EEN-treated CD patients.
| CS | EEN | p-Value | |
|---|---|---|---|
| Anatomical extension, n [%]a | 0 [0.0] | 8 [26.7] | 0.08 |
| Onset of perianal disease, n [%]b | 1 [3.2] | 5 [7.0] | 0.66 |
| Onset of stricturing behaviour, n [%]c | 2 [5.7] | 6 [8.2] | 1.00 |
| Hospitalisation, n [%] | 8 [22.9] | 11 [14.8] | 0.42 |
| Surgery, n [%] | 2 [5.7] | 3 [4.1] | 0.66 |
CS, corticosteroid; EEN, exclusive enteral nutrition; CD, Crohn’s disease.
aPatients with maximal disease extension at diagnosis and/or did not undergo follow-up endoscopic/radiological assessments were not included.
bPatients with perianal disease at diagnosis were not included.
cOne patient presenting with stricturing behaviour at diagnosis was not included.
Almost all patients in our study cohort exhibited inflammatory [B1: non-stricturing, non-penetrating] disease behaviour at diagnosis; only one patient in the EEN group [1.3%] exhibited stricturing [B2] behaviour. Of the remaining patients who could therefore progress from inflammatory behaviour and had ≥ 2 years’ follow-up information available [n = 108], onset of stricturing behaviour occurred in 2/35 [5.7%] CS patients and 6/73 [8.2%] EEN patients [Table 3]. No patients exhibited penetrating disease [B3] by maximum follow-up.
Few patients presented with perianal disease at diagnosis [Table 1]. An additional six patients developed perianal disease by maximum follow-up, but the incidence did not differ significantly between CS- and EEN-treated groups [3.2% versus 7.0%, p = NS] [Table 3].
3.6. Hospitalisations and surgeries
In total, 8/35 [22.9%] CS and 10/74 [13.5%] EEN patients with ≥ 2 years’ follow-up were hospitalised for CD-related complications at least once [p = NS] [Table 3]. Repeated hospitalisations were uncommon, with only two patients [both from the EEN group] being hospitalised two or more times.
There was no significant difference in the proportion of patients undergoing CD-related surgery. The incidence of surgery was relatively rare in our cohort, with only five patients in total [two CS and three EEN] undergoing surgery by maximum follow-up [p = NS] [Table 3]. Surgeries performed in the CS group included ileocecal resection and primary anastomosis, and total colectomy. Surgeries performed in the EEN group included two ileocecal resections and one surgical draining of a perianal abscess.
4. Discussion
This is the first study examining both short- and long-term outcomes of EEN and CS induction therapy in a propensity-score matched paediatric CD cohort. Our findings add further support that EEN is a highly efficacious induction therapy and demonstrate that EEN is associated with long-term steroid avoidance, without increased risk for complications [including surgery] and use of biologics.
Our data show that clinical remission after EEN is superior to that after CS, with 86.6% versus 58.1% of patients reaching remission based on PCDAI score ≤ 7.5 within 4–12 weeks of starting treatment. These results compare favourably with those of the recent European GROWTH CD study, which reported 71% EEN patients versus 46% CS patients achieving remission by 12 weeks.7 Meta-analyses of EEN in paediatric CD patients report clinical response rates from 60 to 85%,27,28 but remission rates as high as 92% have also been reported.29 Consistent with observations in the GROWTH CD study, the early introduction of immunomodulators in our cohort [which was more common in the CS group] had no clear effect on the rate of remission within CS- or EEN-treated groups by 4–12 weeks.7
In addition to demonstrating a high remission rate, patients treated with EEN had the benefit of early growth improvement. Impaired growth is an important complication of CD, specific to the paediatric population.30 Within our study cohort, 12.6% of patients exhibited linear growth impairment [< -1.65 height z-score] at diagnosis, which falls within the 4% to 38% range of growth failure rates reported among paediatric CD patients at time of diagnosis, depending on the definition used.31,32 Despite PS matching, patients in the EEN group presented with lower height z-scores and higher frequency growth failure relative to the CS group, and had significantly lower BMI z-scores, which suggests that nutritional and growth-related concerns factor heavily into patient/clinician decisions to choose EEN for induction therapy. We observed that patients treated with EEN exhibited significantly greater improvement in height z-scores than CS-treated patients by 1-year follow-up, although this effect was not sustained over the 6-year follow-up period of observation. EEN has previously been demonstrated to be more effective than CS in inducing linear growth recovery in studies with follow-up periods ranging from 8 weeks to 3 months,5,33 but there are limited data on the long-term effects of EEN induction therapy on growth. Consistent with our observations, significant [albeit small] improvements in height z-score at 1 and 2 years have been reported in patients treated with EEN for induction compared with patients treated with CS.28,34 The early substantial improvement in height gains of EEN patients may be a reflection of clinical response. Linear growth patterns correlate with disease activity, and there is strong pathophysiological evidence that inflammation interferes with the growth hormone axis.31,35,36 EEN downregulates proinflammatory cytokines including TNF-α and IL-6, after which an increase in growth hormones [IGF-1 and IGFBP-3] is observed within 2 weeks of treatment.37 Thus, our results further support that the combination of nutritional supplementation and anti-inflammatory effect of EEN is associated with short-term improvement in linear growth relative to CS therapy.8 Importantly, EEN may also influence growth recovery in paediatric CD patients by limiting chronic CS exposure, which is a significant contributing factor toward growth failure.
It has been suggested that although EEN is an effective substitute for CS as an induction therapy, EEN results in only a short-term delay in the inevitable use of CS.38 Our study and others support a more optimistic view towards EEN as an approach to CS avoidance: over 40% of EEN-treated patients in our cohort remained steroid-naïve for at least 4 years, and a recent study reported that > 60% EEN-treated children avoid CS completely over a 2-year period.28 Factors contributing to steroid avoidance are unclear. Patients may be inclined to return to familiar therapies to treat disease flares, as was observed in a recent study wherein 82% of children treated with EEN for induction subsequently elected EEN for relapse.39,40 In addition, the choice of EEN as an induction therapy at the outset may reflect a strong desire by patients/patient families to avoid the use of CS. Steroid dependency is a frequent complication in paediatric CD. A large paediatric cohort study of steroid-treated patients showed that a significant portion of steroid dependence [< 25%] is associated with younger age at diagnosis [< 10.7 years] and co-existing upper GI tract involvement, suggesting that children with these features could be potentially targeted for steroid-sparing therapies such as EEN.41 Moreover, concomitant use of immunomodulators was associated with reduced risk of steroid dependency in this cohort.41 Clearly, a logical approach to avoid steroid dependence may be to choose an alternative to CS as induction therapy.
Our study shows that long-term steroid avoidance via EEN is feasible without increased need for escalation to anti-TNF therapy. The majority of patients in the EEN-treated group who remained steroid-naive by 2 and 4 years had also not been exposed to anti-TNF, indicating that early anti-TNF use could account for only a minor portion of steroid avoidance in this group.42 Most patients in our cohort [and in both EEN and CS groups] had moderate to severe active CD [PCDAI ≥ 30] at diagnosis, and both groups required escalation of therapy [44% versus 60% from EEN and CS groups, respectively] by the maximum follow-up. We observed a trend towards delayed escalation to anti-TNF in the EEN group by 2 years, but this was not statistically significant. This slight delay in introducing anti-TNF may relate to the superior ability of EEN to induce early mucosal healing, and could suggest that less severe ongoing disease in the EEN-treated group postpones the need for more aggressive therapy.9,43
We observed a change in disease location, namely extension towards the ileum, in a subset [n = 7] of EEN-treated patients. Previous paediatric cohort studies have reported extension toward ileal disease in approximately 20% of childhood-onset CD patients,2 and suggest that delayed ileal involvement in patients presenting with isolated colonic disease [L2] is a feature of paediatric CD that reflects age at onset.44 L2 disease is a relatively rare phenotype within paediatric CD,2 and the numbers of patients with an L2+/-L4 phenotype in this study are small [n = 9 CS, n = 12 EEN]. Due to the limited sample size of patients eligible to exhibit ileal extension, and the lack of follow-up endoscopies/imaging investigations, we interpret the apparent lack of disease extension in CS-treated patients as a false-negative effect.
The current study is limited by the retrospective design. The comparative groups were matched based on propensity scores and not allocated randomly; therefore, it is possible that unknown variables contribute to patient stratification beyond the PS-matching criteria. Various factors, including patient choice, could contribute to the use of one therapy over another. Furthermore, whereas this study is adequately powered to replicate findings from the GROWTH CD study, the sample size is not large enough to avoid type II error for rare outcomes. Our cohort exhibited a low rate of adverse long-term outcomes, including hospitalisation or surgical resection, which is likely a reflection of the very low incidence of complicated disease at presentation [< 1%]. That said, a recent study of 1- and 2-year outcomes following EEN versus CS induction therapy, also reported no difference in early surgical outcomes between groups.45
In conclusion, despite limitations related to the retrospective study design, the current study demonstrates that the use of EEN as initial induction therapy leads to high rates of remission, improved linear growth, and long-term avoidance of steroids. Our data do not suggest that these outcomes are achieved due to greater use of anti-TNF. More studies are needed to identify which patients will benefit most from early treatment escalation versus those who will benefit most over the long-term from nutrition-focused intervention.
Funding
This work was supported by a Nova Scotia Health Research Foundation [NSHRF] establishment award [JVL] and a Canadian Institutes of Health Research [CIHR] New Investigator Award [201412XGP-340307-205026][JVL], and an IWK Health Centre Research Associateship grant [JC]. JVL and ARO are supported by a CIHR-SPOR-Chronic Diseases grant [Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects: the IMAGINE-SPOR chronic disease network]. SB was supported by a North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition [NASPGHAN] Foundation Mentored Studentship Award [2015].
Conflict of Interest
JVL and ARO have received support for research, development of educational materials, and participation in advisory boards for Nestlé.
Author Contributions
JVL and ARO designed the study. JC, SB, and NG contributed to data collection. JC, AG, and SB analysed the data. JC drafted the manuscript; AG, SB, NG, AN, GM, MR, ARO, and JVL performed a critical revision of the manuscript. JVL obtained funding for the study.
References
- 1. Vernier–Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology 2008;135:1106–13. [DOI] [PubMed] [Google Scholar]
- 2. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
- 3. Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953–61. [DOI] [PubMed] [Google Scholar]
- 4. Malik S, Mason A, Bakhshi A, et al. Growth in children receiving contemporary disease specific therapy for Crohn’s disease. Arch Dis Child 2012;97:698–703 [DOI] [PubMed] [Google Scholar]
- 5. Berni Canani R, Terrin G, Borrelli O, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis 2006;38:381–7. [DOI] [PubMed] [Google Scholar]
- 6. Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2007:CD000542. [DOI] [PubMed] [Google Scholar]
- 7. Levine A, Turner D, Pfeffer Gik T, et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: evaluation of the Porto IBD group “growth relapse and outcomes with therapy” [GROWTH CD] study. Inflamm Bowel Dis 2014;20:278–85. [DOI] [PubMed] [Google Scholar]
- 8. Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr 2000;31:8–15. [DOI] [PubMed] [Google Scholar]
- 9. Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 2006;4:744–53. [DOI] [PubMed] [Google Scholar]
- 10. Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther 2007;26:795–806. [DOI] [PubMed] [Google Scholar]
- 11. Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol 2014;49:638–45. [DOI] [PubMed] [Google Scholar]
- 12. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 13. Levine A, Wine E. Effects of enteral nutrition on Crohn’s disease: clues to the impact of diet on disease pathogenesis. Inflamm Bowel Dis 2013;19:1322–9. [DOI] [PubMed] [Google Scholar]
- 14. Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr 2011;52:38–42. [DOI] [PubMed] [Google Scholar]
- 15. Van Limbergen J, Haskett J, Griffiths AM, et al. Toward enteral nutrition for the treatment of pediatric Crohn disease in Canada: a workshop to identify barriers and enablers. Can J Gastroenterol Hepatol 2015;29:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 17. McNamee R. Regression modelling and other methods to control confounding. Occup Environ Med 2005;62:500–6, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. WHO AnthroPlus for Personal Computers. Manual: Software for Assessing Growth of the World’s Children and Adolescents. Geneva: WHO; 2011. [Google Scholar]
- 20. Turner D, Griffiths AM, Walters TD, et al. Appraisal of the pediatric Crohn’s disease activity index on four prospectively collected datasets: recommended cutoff values and clinimetric properties. Am J Gastroenterol 2010;105:2085–92. [DOI] [PubMed] [Google Scholar]
- 21. Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47. [PubMed] [Google Scholar]
- 22. Hyams J, Markowitz J, Otley A, et al. Pediatric Inflammatory Bowel Disease Collaborative Research Group. Evaluation of the Pediatric Crohn Disease Activity Index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr 2005;41:416–21. [DOI] [PubMed] [Google Scholar]
- 23. Gilbert GE, Prion S. Making sense of methods and measurement: the danger of the retrospective power analysis. Clin Simul Nurs 2016;12:303–4. [Google Scholar]
- 24. Thoemmes F. Propensity score matching in SPSS. arXiv preprint arXiv 2012:12016385. [Google Scholar]
- 25. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R. Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med 2010;36:1993–2003. [DOI] [PubMed] [Google Scholar]
- 27. Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther 2008;27:293–307. [DOI] [PubMed] [Google Scholar]
- 28. Lambert B, Lemberg DA, Leach ST, Day AS. Longer-term outcomes of nutritional management of Crohn’s disease in children. Dig Dis Sci 2012;57:2171–7. [DOI] [PubMed] [Google Scholar]
- 29. Frivolt K, Schwerd T, Werkstetter KJ, et al. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: predictors of efficacy and outcome. Aliment Pharmacol Ther 2014;39:1398–407. [DOI] [PubMed] [Google Scholar]
- 30. Sanderson IR. Growth problems in children with IBD. Nat Rev Gastroenterol Hepatol 2014;11:601–10. [DOI] [PubMed] [Google Scholar]
- 31. Ley D, Duhamel A, Behal H, et al. Growth pattern in paediatric Crohn disease is related to inflammatory status. J Pediatr Gastroenterol Nutr 2016;63:637–43. [DOI] [PubMed] [Google Scholar]
- 32. Vasseur F, Gower-Rousseau C, Vernier-Massouille G, et al. Nutritional status and growth in pediatric Crohn’s disease: a population-based study. Am J Gastroenterol 2010;105:1893–900. [DOI] [PubMed] [Google Scholar]
- 33. Azcue M, Rashid M, Griffiths A, Pencharz PB. Energy expenditure and body composition in children with Crohn’s disease: effect of enteral nutrition and treatment with prednisolone. Gut 1997;41:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papadopoulou A, Rawashdeh MO, Brown GA, McNeish AS, Booth IW. Remission following an elemental diet or prednisolone in Crohn’s disease. Acta Paediatr 1995;84:79–83. [DOI] [PubMed] [Google Scholar]
- 35. D’Mello S, Trauernicht A, Ryan A, et al. Innate dysfunction promotes linear growth failure in pediatric Crohn’s disease and growth hormone resistance in murine ileitis. Inflamm Bowel Dis 2012;18:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeBoer MD, Scharf RJ, Leite AM, et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition 2017;33:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn’s disease: pathogenesis and interventions. Inflamm Bowel Dis 2007;13: 620–8. [DOI] [PubMed] [Google Scholar]
- 38. Murphy M, Randell T. Evaluation of elemental diet therapy as a long-term strategy for managing Crohn’s disease. Arch Dis Child 2001;84:A4. [Google Scholar]
- 39. Grogan JL, Casson DH, Terry A, Burdge GC, El-Matary W, Dalzell AM. Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: a double-blind randomized controlled trial with two years follow-up. Inflamm Bowel Dis 2012;18:246–53. [DOI] [PubMed] [Google Scholar]
- 40. Knight C, El-Matary W, Spray C, Sandhu BK. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin Nutr 2005;24:775–9. [DOI] [PubMed] [Google Scholar]
- 41. Krupoves A, Mack DR, Seidman EG, Deslandres C, Bucionis V, Amre DK. Immediate and long-term outcomes of corticosteroid therapy in pediatric Crohn’s disease patients. Inflamm Bowel Dis 2011;17:954–62. [DOI] [PubMed] [Google Scholar]
- 42. Kane SV, Jaganathan S, Bedenbaugh AV, Palmer L, Schwartz DA. Anti-tumor necrosis factor agents reduce corticosteroid use compared with azathioprine in patients with Crohn’s disease. Curr Med Res Opin 2014;30:1821–6. [DOI] [PubMed] [Google Scholar]
- 43. Grover Z, Burgess C, Muir R, Reilly C, Lewindon PJ. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J Crohns Colitis 2016;10:1159–64. [DOI] [PubMed] [Google Scholar]
- 44. Meinzer U, Ideström M, Alberti C, et al. Ileal involvement is age dependent in pediatric Crohn’s disease. Inflamm Bowel Dis 2005;11:639–44. [DOI] [PubMed] [Google Scholar]
- 45. Grover Z, Lewindon P. Two-year outcomes after exclusive enteral nutrition induction are superior to corticosteroids in pediatric Crohn’s disease treated early with thiopurines. Dig Dis Sci 2015;60:3069–74. [DOI] [PubMed] [Google Scholar]