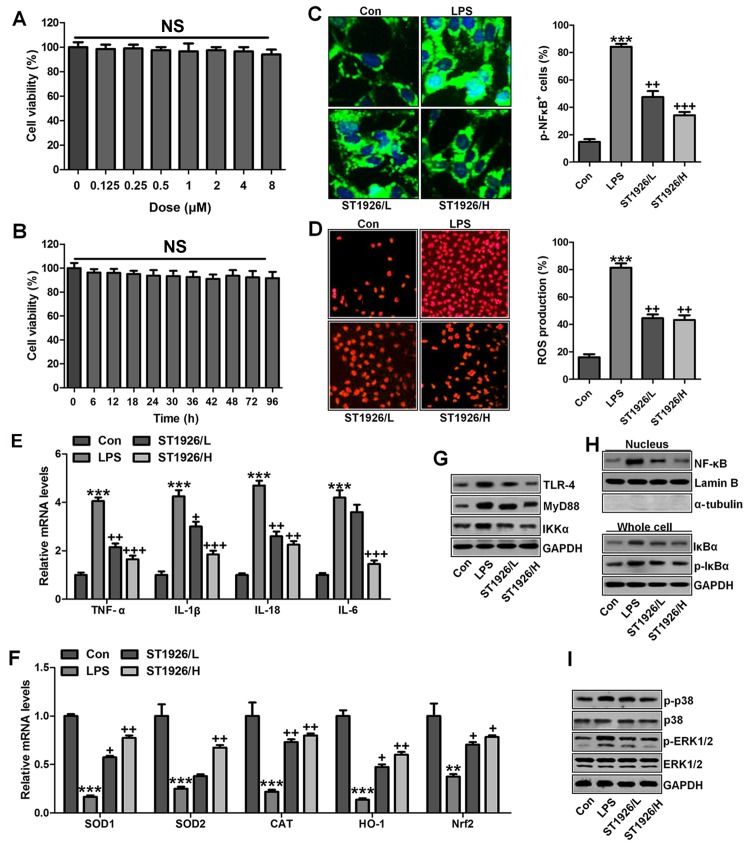
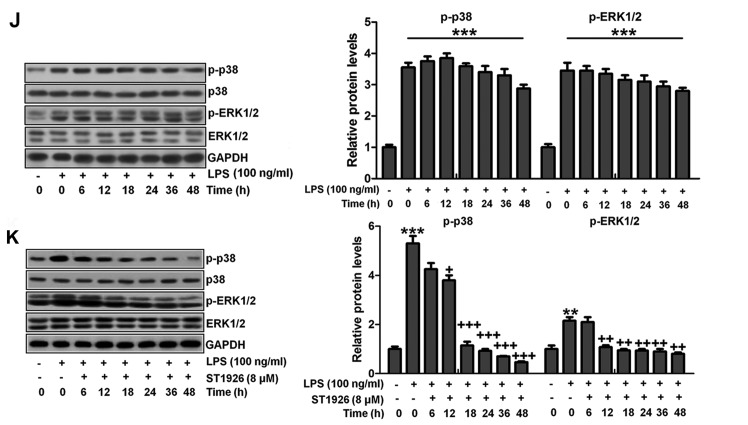
Figure 12.
ST1926 inhibits inflammation response and reactive oxygen species (ROS) generation in human bronchial epithelial cells. (A) Human bronchial epithelial cells were treated with different concentrations of ST1926 at different concentrations for 24 h. Then, the cell viability was calculated through MTT analysis. (B) Human bronchial epithelial cells were treated with 8 µM ST1926 for different time, followed by MTT assays. (C) The phosphorylated nuclear factor-κB (NF-κB) levels were measured by immunofluorescent analysis. (D) ROS generation was evaluated and the quantification was displayed. (E) RT-qPCR assays were performed to determine tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-18 and IL-6 gene levels in cells under different treatment. (F) SOD1, SOD2, CAT, haeme oxygenase-1 (HO-1) and Nrf2 mRNA levels were calculated through RT-qPCR assays. (G) Toll-like receptor 4 (TLR4), MyD88 and inhibitor-κB kinase (IKKα) protein expression levels were measured by western blot analysis. (H) Western blotting was carried out to investigate nuclear NF-κB levels. The cytoplasm p-inhibitor-κB kinase-α (IκBα) and IκBα levels were assessed through western blot analysis. (I) Phosphorylated p38, and (H) extracellular receptor kinase 1/2 (ERK1/2) was determined by western blot analysis. (J) NHBE cells were exposed to 100 ng/ml lipopolysaccharide (LPS) for different times as indicated, followed by western blot analysis. (K) ST1926 reduced p38 and ERK1/2 phosphorylation in human bronchial epithelial cells after LPS treatment with or without ST1926 administration from 0 to 48 h. Then, western blot assays were used to explore p38 and ERK1/2 activation. The data are presented as mean ± SD (n=8). *p<0.05 and **p<0.001 vs. the control (Con); +p<0.05, ++p<0.01 and +++p<0.001 vs. LPS-induced mice (LPS).