Abstract
Background:
The presence of asymptomatic spinal cord (SC) lesions in patients with clinically isolated syndrome (CIS) or relapsing-remitting multiple sclerosis (RRMS) predicts conversion to clinically definite multiple sclerosis (CDMS). The relation between asymptomatic SC abnormalities and disability progression warrants further investigation.
Objective:
To determine the prognostic value of asymptomatic SC lesions in CIS and early RRMS with respect to the time to disability development.
Methods:
Clinical and demographic data, brain and SC magnetic resonance imaging (MRI) were collected of CIS or early RRMS patients. Two main analyses were performed. For the first analysis, patients were divided into two groups: (1) patients with asymptomatic SC lesions and (2) patients without SC lesions and patients with symptomatic SC lesions. The second analysis excluded patients with symptomatic SC lesions. Incidence curves were used to analyse differences between these groups in time to the development of disability and time to a second relapse.
Results:
A total of 178 patients were included, and 42 patients (23.6%) had asymptomatic SC lesions. No significant differences were found on the time to disability development or the time to a second event.
Conclusion:
Asymptomatic SC lesions early in the disease course do not predict the time to disability development in patients diagnosed with CIS or early RRMS.
Keywords: Clinically isolated syndrome, multiple sclerosis, disability, magnetic resonance imaging, asymptomatic spinal cord lesions, progression
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease of the central nervous system, affecting the brain as well as the spinal cord (SC).1 The vast majority of patients have a relapsing-remitting disease course of MS (RRMS), typically starting with a first clinical episode: the clinically isolated syndrome (CIS).1,2 Over years, magnetic resonance imaging (MRI) has gained more weight in the diagnostic process according to diagnostic criteria and may have value regarding disease monitoring and understanding disease progression.3–6 SC imaging may be decisive in diagnosing MS; in case a brain MRI cannot confirm the diagnosis, SC imaging can result in completion of the diagnosis especially if an asymptomatic SC lesion is present.3,7
The recently published magnetic resonance imaging in multiple sclerosis (MAGNIMS) guidelines on MRI in the diagnostic process of MS recommend SC imaging in patients with SC symptoms at onset, in case of inconclusive or ambiguous brain MRI and in case of radiologically isolated syndrome (RIS).4,5 SC imaging is not recommended for monitoring MS disease and treatment. However, the presence of asymptomatic SC lesions as the first symptoms manifest or during the early disease course of RRMS also deserve special attention in light of predicting future disability and relapses.7–11
Asymptomatic SC lesions are frequently reported in patients with a CIS ranging from 27% to 53% in different studies10,12,13 and new asymptomatic SC lesions are reported in 25% of patients with clinically stable RRMS.11 Different studies demonstrate that the presence of asymptomatic SC lesions predicts conversion to clinically definite multiple sclerosis (CDMS) in CIS patients.7,9,10,14 Moreover, Asymptomatic SC lesions increase the risk for relapses7,11,15 and disability.10,14,16
We hypothesize that advances in knowledge with respect to the prognostic impact of SC lesions can lead to a better counselling on MS disease, as well as treatment decisions. Therefore, it is important to not only know whether a future event will occur, but especially how soon this may happen. The aim of this study was to determine the prognostic value of asymptomatic SC lesions on the time to disability and a second relapse in a group of patients with a CIS or an early course of RRMS.
Methods
Patients
This longitudinal cohort study included patients from the Amsterdam Multiple Sclerosis Cohort. Patients were recruited from December 2000 until September 2007 and included when diagnosed with a CIS or an early course of RRMS (within 12 months after the first symptoms). Details on this cohort have been described previously.7
CIS or early MS according to the McDonald criteria 2010 was diagnosed in 293 patients. We defined the following inclusion criteria:
Age at onset of first symptoms between 16 and 60 years old (4 patients excluded);
A clinical follow-up (FU) of at least 24 months (15 patients excluded);
A baseline visit within 12 months after disease onset (81 patients excluded);
Both brain MRI and SC MRI available at baseline or within 3 months of the baseline visit (15 patients excluded);
Patients with asymptomatic SC lesions or patients without SC lesions. Patients with symptomatic SC lesions were excluded for subanalysis (84 patients excluded).
Written informed consent was obtained from all participants. The local institutional review board approved the data acquisition of this Amsterdam Multiple Sclerosis Cohort and this particular study.
Clinical assessments
Patients were followed regularly for at least 2 years up to 11 years. Location of symptom onset (optic nerve, SC, brainstem/posterior fossa, hemispherical) and location of relapse symptoms, if prior to the baseline visit, were recorded, also in case of multifocal neurological symptoms. Clinical data further included disease-modifying therapy (DMT), relapses, location and recovery of relapses between the visits at baseline, 3 months, 1 and 2 years or when not lost to FU, also at 5, 6 and 11 years.
Diagnosis of RRMS was made according to the 2010 revision of the McDonald criteria.3 These diagnostic criteria were not available at the baseline and first FU visits; therefore, we retrospectively applied these criteria using clinical and radiological data. In doing so, 50 patients originally regarded as being CIS7 now are labelled as RRMS.
Expanded Disability Status Scale (EDSS) scores were obtained by certified raters. EDSS collected by telephone was excluded for analyses. Confirmed EDSS progression was defined as reaching twice a score of 3 or 6, with at least 3 months between the two visits. Disability progression was further defined as a 20% increase in time on the timed 25-Foot Walk Test (25-FWT) and 9-hole peg test (9-HPT) with confirmation at least 3 months later.
In order to evaluate the possible effect of DMT use, we obtained information about DMT use at baseline and during FU and calculated whether patients started DMT before reaching the different endpoints.
Neuroimaging
At baseline, 3 months and 2 year FU, brain and SC MRI including post-gadolinium series were obtained. Brain MRI was also performed after 1- and 5-year FU for most patients. These scans were performed on a 1.0 T or 1.5 T whole-body MR systems (Magnetom Impact Expert, Vision, and Sonata Siemens AG, Erlangen, Germany) as described previously.7 Brain MRI was performed using standard two-dimensional, dual-echo, spin-echo, proton-density and T2-weighted images (repetition time (TR): 2200–3000 milliseconds; echo time (TE): 20–30 and 80–100 milliseconds) and T1-weighted images after administration of intravenous gadolinium (TR:500–600 milliseconds; TE: 20 milliseconds), slice thicknesses of 3–5 mm, with a 10% gap and an in-plane resolution of 1 × 1 mm2. A blinded and experienced radiology reader (Image Analysis Center), unaware of the patients’ condition and with more than 10 years of experience, identified lesions on T2-weighted and T1-weighted post-gadolinium images. Lesions were categorized according to their location: juxtacortical, periventricular, deep white matter, corpus callosum, basal ganglia, internal capsule, brainstem, cerebellum and SC. SC imaging included a cardiac-triggered sagittal proton-density and T2-weighted, dual-echo, spin-echo sequence with a slice thickness of 3 mm (TR: 2500–3000 milliseconds; TE: 20–30 and 80–100 milliseconds), with a 0.3-mm gap between slices, and an in-plane resolution of 1 × 1 mm2, using spinal phased-array coils. Imaging covered the full length of the SC. An experienced reader (Image Analysis Center), unaware of the patients’ clinical condition, scored the SC T2 lesions and gadolinium-enhancing lesions.
MRI was performed preferably on the visit date, but at least within 3 months prior or after the baseline clinical assessments.
Statistical analysis
To evaluate the influence of asymptomatic SC lesions on the different outcome measures, we performed two analyses. For the first analysis, the patient population was divided into two groups based on the presence of asymptomatic SC lesions on baseline MRI: (1) patients with asymptomatic SC lesions and (2) patients without SC lesions or patients with SC symptoms. Patients with a SC syndrome and one or more lesions in the SC were defined as patients with symptomatic SC lesions and subdivided to the second group. We also performed analysis excluding patients with symptomatic SC lesions.
Visual inspection of histograms was performed to evaluate normality of the variables. Descriptive statistics were calculated for the total group of patients and the subgroups based on the presence of asymptomatic SC lesions. Groups were compared using independent samples t-test or Mann–Whitney U test as appropriate. Discrete variables were compared using the chi-square test.
The time to reach a confirmed EDSS of 3 or 6 was calculated for patients reaching this milestone. If patients showed a confirmed 20% velocity decrease on the 25-FWT or 9-HPT compared to baseline, the time to reach this difference was calculated. In addition, the time to the second clinical relapse was calculated. Patients not reaching the endpoint during FU were censored at their last FU. Kaplan–Meier curves with log-rank tests were used to analyse the time to event between the two different groups. Correction by Cox-regression analyses were performed to correct for diagnosis (CIS/RRMS) at baseline, disease duration at baseline, number of T2 brain lesions and start of DMT before reaching the endpoint. The second comparison (asymptomatic SC lesions vs patients without SC lesions) was also adjusted for T1 gadolinium-enhancing lesions.
Results
Demographic, clinical and radiological characteristics
We included 178 patients of the 293 patients of the Amsterdam Multiple Sclerosis cohort. At baseline, 47.8% had a CIS and 52.2% of the patients were diagnosed with RRMS. Patients were divided into two groups dependent on the presence of asymptomatic SC lesions. Asymptomatic SC lesions were seen in 42 patients (23.6%), 136 patients (76.4%) reported SC symptoms (n = 84), or did not have lesions on SC MRI (n = 52). Table 1 shows the baseline demographic, clinical and MRI characteristics for all patients together and separately per group. Baseline differences between groups were the number of T2 lesions on brain MRI (p = 0.039) as well as T2 lesions on SC MRI (p = 0.003). The percentage of patients who started DMT before reaching an EDSS of 3 and before 20% increase in 25-FWT was significantly higher in the group with asymptomatic SC lesions compared to the group without asymptomatic SC lesions (data not shown). For analysis, excluding patients with symptomatic SC lesions, 94 patients were included, 42 patients with asymptomatic SC lesions and 52 patients without SC lesions. Baseline characteristics of these groups are shown in Table 2. The percentage of patients diagnosed with CIS at baseline was significantly higher in the group without SC lesions at baseline (p = 0.004). The number of T2 and the number of T1 gadolinium-enhancing lesions was significantly higher in the group with asymptomatic SC lesions (respectively p = 0.001 and p = 0.026). The percentage of patients that started DMT before reaching the clinical endpoints was significantly higher in the asymptomatic SC lesions group.
Table 1.
Patient characteristics.
| All patients | Asymptomatic SC lesions | Other patients | Significance p < 0.05 |
|
|---|---|---|---|---|
| Total no. | 178 | 42 | 136 | |
| Symptomatic SC lesions | 84 | |||
| No lesions, but SC symptoms | 20 | |||
| No lesions, nor symptoms | 32 | |||
| CIS/RRMS (CIS, %) | 85 (47.8) | 15 (35.7) | 70 (51.5) | 0.074c |
| Onset symptoms | ||||
| Optic nerve (%) | 30 (16.9) | 12 (28.6) | 18 (13.2) | |
| Brainstem/posterior fossa (%) | 40 (22.5) | 19 (45.2) | 21 (15.4) | |
| Spinal cord (%) | 75 (42.1) | 0 (0) | 75 (55.1) | |
| Hemispherical (%) | 7 (3.9) | 3 (7.1) | 4 (2.9) | |
| Other (%) | 1 (0.6) | 1 (2.4) | 0 (0) | |
| Multifocal (%)1 | 25 (14.0) | 7 (16.7) | 18 (13.2) | |
| Relapse symptoms, if before BL2 | 67 | 15 | 52 | |
| No. of patients with a second relapse before BL | 53 | 9 (21.4) | 44 (32.4) | |
| No. of patients with a third relapse before BL | 14 | 6 (14.3) | 8 (5.9) | |
| Optic nerve (%) | 14 (20.1) | 4 (26.7) | 10 (19.2) | |
| Brainstem/posterior fossa (%) | 16 (23.9) | 7 (46.7) | 9 (17.3) | |
| Spinal cord (%) | 28 (41.8) | 0 (0) | 28 (53.8) | |
| Hemispherical (%) | 4 (6.0) | 2 (13.3) | 2 (3.8) | |
| Other/unknown | 3 (4.5) | 2 (13.3) | 1 (1.9) | |
| Multifocal (%)3 | 2 (3.0) | 0 (0) | 2 (3.8) | |
| Gender (female, %) | 119 (66.9) | 30 (71.4) | 89 (65.4) | 0.47c |
| Age at onset (years, mean, SD) | 34.8 (8.8) | 34.8 (9.1) | 34.8 (8.7) | 0.94a |
| Disease duration at BL (months, median, IQR) | 6 (3–8) | 5 (3–6) | 6 (4–8) | 0.16b |
| EDSS at BL (median, IQR) | 2.0 (1.5–3.0) | 2.0 (1.4–2.6) | 2.0 (1.5–3.0) | 0.19b |
| 9-HPT (seconds, mean, ±SD) | 18.1 (3.6) (1 missing) |
18.8 (4.7) | 17.9 (3.2) (1 missing) |
0.18a |
| 25-FWT BL (seconds, mean, ±SD) | 4.2 (1.4) (3 missing) |
4.2 (1.2) | 4.2 (1.5) (3 missing) |
0.54a |
| FU length (months, median, IQR) | 72.0 (60.0–125.3) | 83.5 (64.0–127.3) | 70.0 (60.0–124.5) | 0.207b |
| Brain MRI | ||||
| No. of T2 brain lesions (median, IQR) | 12.5 (7.0–26.8) | 17.0 (7.8–28.0) | 11.5 (6.0–22.5) | 0.039b |
| No. of abnormal brain MRI (T2 lesions, %) | 169 (96) | 42 (100) | 127 (95) | 0.13c |
| No. of T1 Gd+ brain lesions (median, IQR) | 0 (0–1) | 0.5 (0–2) | 0 (0–1) | 0.16b |
| No. of abnormal brain MRI (T1 Gd+ lesions, %) | 71 (40) (3 missing) |
21 (50) | 50 (38) (3 missing) |
0.15c |
| SC MRI | ||||
| No. of T2 SC lesions (median, IQR) | 1 (0–4) | 2 (1–4) | 1 (0–3.75) | 0.003b |
| No. of T1 Gd+ SC lesions (median, IQR) | 0 (0–0) | 0 (0–0.25) | 0 (0–0) | 0.46b |
SC: spinal cord; CIS: clinically isolated syndrome; RRMS: relapsing-remitting multiple sclerosis; BL: baseline; SD: standard deviation; IQR: interquartile range; EDSS: Expanded Disability Status Scale; 9-HPT: 9-hole peg test; 25-FWT: 25-Foot Walk Test; FU: follow-up; MRI: magnetic resonance imaging.
12 optic nerve, 19 brainstem/posterior fossa, 15 spinal cord, 4 hemispherical.
The sum of both 2nd and 3rd relapse symptom localisation.
1 Optic nerve, 1 brainstem/posterior fossa, 2 spinal cord.
Independent Samples T-test.
Mann-Whitney U-test.
Chi-square.
Table 2.
Patient characteristics (exclusion of patients with symptomatic SC lesions).
| Total | Asymptomatic SC lesions | No SC lesions | Significance p < 0.05 |
|
|---|---|---|---|---|
| Total no. | 94 | 42 | 52 | |
| No lesions, but SC symptoms | 20 | |||
| No lesions, nor symptoms | 32 | |||
| CIS/RRMS (CIS, %) | 49 (52.1) | 15 (35.7) | 34 (65.4) | 0.004c |
| Onset symptoms | ||||
| Optic nerve (%) | 22 (23.4) | 12 (28.6) | 10 (19.2) | |
| Brainstem/posterior fossa (%) | 38 (40.4) | 19 (45.2) | 19 (36.5) | |
| Spinal cord (%) | 11 (11.7) | 0 (0) | 11 (21.2) | |
| Hemispherical (%) | 6 (6.4) | 3 (7.1) | 3 (5.8) | |
| Other (%) | 1 (1.1) | 1 (2.4) | 0 (0) | |
| Multifocal (%)1 | 16 (17.0) | 7 (16.7) | 9 (17.3) | |
| Relapse symptoms, if before BL2 | 27 | 15 | 12 | |
| No. of patients with a second relapse before BL | 19 | 9 | 10 | |
| No. of patients with a third relapse before BL | 8 | 6 | 2 | |
| Optic nerve (%) | 8 (29.6) | 4 (26.7) | 4 (33.3) | |
| Brainstem/posterior fossa (%) | 8 (37.0) | 7 (46.7) | 3 (25.0) | |
| Spinal cord (%) | 2 (7.4) | 0 (0) | 2 (16.7) | |
| Hemispherical (%) | 4 (14.8) | 2 (13.5) | 2 (16.7) | |
| Other/unknown | 2 (7.4) | 2 (13.5) | 0 (0) | |
| Multifocal (%)3 | 1 (3.7) | 0 (0) | 1 (8.3) | |
| Gender (female, %) | 61 (64.9) | 30 (71.4) | 31 (59.6) | 0.23c |
| Age at onset (years, mean, SD) | 35.0 (8.4) | 34.8 (9.1) | 35.1 (8.0) | 0.34a |
| Disease duration at BL (months, median, IQR) | 5 (3–7) | 5 (3–6) | 5 (3–7) | 0.72b |
| EDSS at BL (median, IQR) | 2.0 (1.5–3.0) | 2.0 (1.4–2.6) | 2.3 (1.5–3.8) | 0.35b |
| 9-HPT (seconds, mean, ±SD) | 18.2 (3.6) | 18.8 (4.7) | 17.7 (2.5) | 0.15a |
| 25-FWT BL (seconds, mean, ±SD) | 4.0 (1.2) | 4.2 (1.2) | 3.9 (1.3) | 0.78a |
| FU length (months, median, IQR) | 71.0 (58.0–127.3) | 83.5 (64.0–127.3) | 66.5 (56.3–127.3) | 0.050b |
| Brain MRI | ||||
| No. of T2 brain lesions (median, IQR) | 12.5 (7.0–21.3) | 17.0 (7.8–28.0) | 9.0 (4.3–16.0) | 0.001b |
| No. of abnormal brain MRI (T2 lesions, %) | 91 (96.8) | 42 (100) | 49 (94.2) | 0.11c |
| No. of T1 Gd+ brain lesions (median, IQR) | 0 (0–1) | 0.5 (0–2) | 0 (0–1) | 0.026 |
| No. of abnormal brain MRI (T1 Gd+ lesions, %) | 37 (39.4) | 21 (50.0) | 16 (30.8) | 0.058c |
| SC MRI | ||||
| No. of T2 SC lesions (median, IQR) | 0 (0–2) | 2 (1–4) | – | – |
| No. of T1 Gd+ SC lesions (median, IQR) | 0 (0–0) | 0 (0–0.3) | – | – |
SC: spinal cord; CIS: clinically isolated syndrome; RRMS: relapsing-remitting multiple sclerosis; BL: baseline; SD: standard deviation; IQR: interquartile range; EDSS: Expanded Disability Status Scale; 9-HPT: 9-hole peg test; 25-FWT: 25-Foot Walk Test; FU: follow-up; MRI: magnetic resonance imaging.
9 optic nerve, 13 brainstem/post fossa, 6 spinal cord, 4 hemispherical.
The sum of both 2nd and 3rd relapse symptom localisation.
1 brainstem/posterior fossa, 1 spinal cord.
Independent Samples T-test.
Mann-Whitney U-test.
Chi-square.
Disability development
From the total of 178 patients, during FU 70 patients (39.3%) reached an EDSS of 3, 11 patients (6.2%) reached an EDSS of 6. A total of 40 patients reached a 20% increase on the time on the 25-FWT and 23 patient on the 9-HPT during FU.
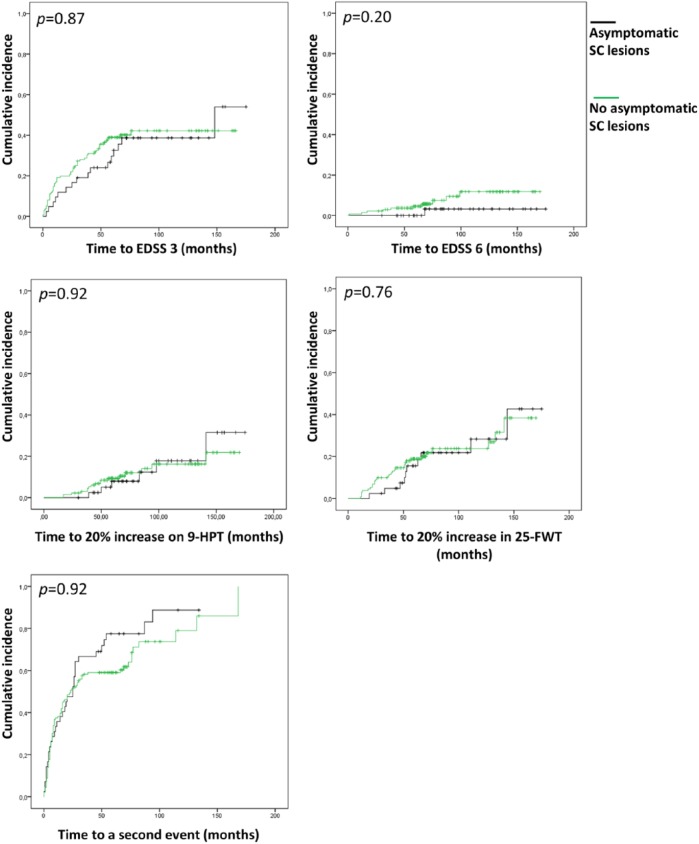
Cumulative incidence curves for all five outcome measures including the results of the log-rank tests after correction with Cox-regression analyses are shown in Figure 1. No significant differences were found for any of the disability outcome measures, as well as no significant differences for the time to a second clinical event.
Figure 1.
Cumulative incidence curves for disability progression and second relapse. Showing the comparison between patients with asymptomatic SC lesions and the patients with symptomatic SC lesions and the patients without SC lesions. Corrected p-values are given. Correction by Cox-regression analyses were performed to correct for diagnosis (CIS/RRMS) at baseline, disease duration at baseline, number of T2 brain lesions and start of DMT before reaching the endpoint.
EDSS: Expanded Disability Status Scale; 9-HPT: 9-hole peg test; 25-FWT: 25-foot walking test.
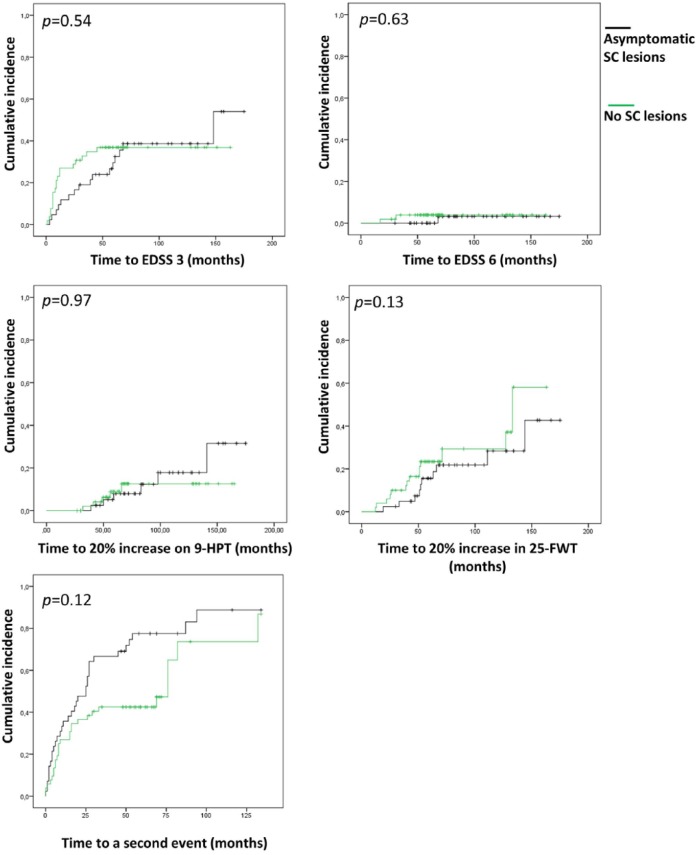
We performed a subanalysis, excluding patients with symptomatic SC lesions. For this analysis, 94 patients were included, of which 35 patients (37.2%) reached an EDSS of 3 during FU and 3 patients (3.2%) reached an EDSS of 6. Cumulative incidence curves for this subanalysis including the log-rank tests after correction with Cox-regression analyses are shown in Figure 2.
Figure 2.
Cumulative incidence curves for disability progression and second relapse. Showing the comparison between patients with asymptomatic SC lesions and the patients without SC lesions. Corrected p-values are given. Correction by Cox-regression analyses were performed to correct for diagnosis (CIS/RRMS) at baseline, disease duration at baseline, number of T2 brain lesions, start of DMT before reaching the endpoint and number of T1 gadolinium-enhancing lesions.
EDSS: Expanded Disability Status Scale; 9-HPT: 9-hole peg test; 25-FWT: 25-foot walking test.
Furthermore, subanalyses were performed on CIS patients only. No significant differences were found on the time to disability or a second relapse between the two groups. Besides, we also performed a subanalysis subdividing the group into four groups based on the report of SC symptoms and/or SC lesions at baseline MRI: (1) patients with asymptomatic SC lesions (n = 42), (2) patients with symptomatic SC lesions (n = 84), (3) patients without SC lesions but having SC symptoms (n = 20) and (4) patients not having SC lesions or SC symptoms (n = 32). No significant differences were found comparing these groups.
Discussion
The main aim of this longitudinal cohort study on CIS and early RRMS patients was to investigate the influence of asymptomatic SC lesions on the time to disability or the time to a second relapse. We performed two main analyses, the first one comparing patients with asymptomatic SC lesions to patients without SC lesions and patients with symptomatic SC lesions. For the second analysis, the patients with symptomatic SC lesions were excluded, to compare the group with asymptomatic SC lesions to the group without SC lesions. The most relevant finding of this study suggests no relationship between the presence of asymptomatic SC lesions and the time to disability development in the short to medium length of the disease course.
We performed a subanalysis between four groups based on the presence of SC symptoms and SC lesions, but none of these analyses showed differences in the time to reach the event outcome. Previous studies suggested that asymptomatic SC lesions can predict future disability,10,11,14,16 as well as the risk and the time to clinically definite MS according to previous diagnostic criteria.7,9,10,14 We expected to find a shorter time to a second relapse in the group with asymptomatic SC lesions because this is related to the definition of CDMS (having a second clinical event).17 However, these inconclusive findings across different studies and our results could be explained by differences in patient selection, (statistical) methods, and mostly outcome measures. For example, a study including 121 CIS patients,7 which was largely based on the same cohort of patients, used the diagnostic criteria according to Poser et al.17 We included not only CIS patients but also recently diagnosed RRMS patients according to retrospectively applied McDonald criteria 2010, which resulted in a slightly different population with respect to disease course at baseline. We performed a subgroup analysis, including only CIS patients, but we did not find a difference in the time to disability progression or a second relapse.
A study, mainly focussing on the importance of SC lesions as a predictor for MS diagnosis and for disability progression, also determined the role of asymptomatic SC lesions. This study, including 207 patients, indicates a predictive value of asymptomatic SC lesions for disability progression. However, only a small part of the cohort developed an EDSS of ≥3 (6.3% of the patients) and the follow-up length was quite short (average of about 3 years).14 One study included only patients with an optic neuritis as initial symptom and measured the risk of conversion to CDMS as well as the risk of disability.10 In contrast to that specific study, patients in our cohort had a higher EDSS and the number of asymptomatic SC lesions was noticeably higher. Possibly, this could be explained by a more favourable disease course for patients with an initial optic neuritis with only 12% of the patients having an EDSS score ≥3 after 5 years of FU. Other studies that investigated the prognostic value of asymptomatic SC lesions on disability progression do not agree on the outcomes of our study, but differ in methods as well. A study on 131 CIS patients that only included patients without SC symptoms found baseline SC lesions to be associated with EDSS scores after 5 years of FU.16 Onset symptoms were mostly optic neuritis (87%), which does not reflect the average CIS population and has probably a different future disability perspective than the patients in our cohort. In contrary to studies favouring asymptomatic SC lesions as a predictor, a study on 103 RRMS patients did not find a difference in the risk of disability progression for patients with asymptomatic SC lesions.11 This last mentioned study included patients with a stable disease during first (baseline) and second (years 1–3) of the FU, which could have filtered out the patients developing disability in the meantime.
A possible limitation of our study is that we included patients with a follow-up of at least 2 years, some patients were not followed for a longer period than these 2 years, and possibly it was too early in the disease course to detect disability progression. Furthermore, some patients who were still CIS at 6 years of follow-up were not yet seen again at the 11-year FU moment. Moreover, some endpoints were only reached by a low number of patients. Larger studies, with even longer FU, could overcome this limitation.
Besides the SC lesions, also other SC measures have been shown to influence disability. For example, SC atrophy was related to disability progression in a study following 131 patients for about 5 years.16 Moreover, upper cervical SC atrophy is associated with disability in cross-sectional studies.18,19 Not only atrophy but also diffuse demyelinating cervical SC lesions predicted disability progression in a small group of patients.20 We did not account for SC atrophy or diffuse demyelinating SC lesions in this study.
In addition to the fact that the acquisition and interpretation of SC images is rather challenging, it is important to bear in mind that asymptomatic SC lesions are difficult to distinguish from SC lesions that are not symptomatic, so some patients have both symptomatic and asymptomatic SC lesions, which makes it difficult to investigate the real prognostic value of asymptomatic SC lesions on disability progression.
In conclusion, previous studies have suggested asymptomatic SC lesions to predict future disability at a certain moment during follow-up, but the time to the development of disability was not measured before. To answer this question, we used a large natural history cohort, followed for a long period and measured different disability outcome parameters. Our results suggest that asymptomatic SC lesions do not predict the time to disability development. The role of asymptomatic SC lesions with regard to the prediction of patient outcome remains enigmatic. Given the inconclusive data on the role of asymptomatic SC lesions on clinical outcome measures, future research with longer follow-up and possibly larger cohorts is needed.
Acknowledgments
The authors thank Dr L. van Winsen, Dr M.J. Eikelenboom, Dr J.J. Kragt, Dr B. Jasperse, Dr T. Korteweg, Dr A. Minneboo, Dr L.V.A.E. Bosma, Dr L.J. Balk, Dr L.F. van der Voort and the research assistants for their assistance with data collection. The authors also thank Tineke van IJken (Image Analysis Centre) for her support in analysing the MRI scans. Finally, the authors thank all subjects for participating in this study. This research has been executed within the VUmc MS Centre Amsterdam.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: I.D., M.H.S. and B.I.W. do not report any competing interests. J.J.G.G. serves on the editorial boards of Multiple Sclerosis Journal, BMC Neurology, MS International and Neurology and the Scientific Advisory Board of the Dutch MS Research Foundation and of MS Academia, Merck Serono and has served as a consultant for Merck Serono, Biogen Idec, Novartis, Genzyme and Teva Pharmaceuticals. B.M.J.U. has received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and Teva. F.B. serves as a consultant for Bayer-Schering Pharma, Sanofi-Aventis, Biogen, Teva, Novartis, Roche, Synthon BV, Genzyme and Jansen Research. J.K. has received consultancy fees from Merck Serono, Teva, Biogen, Genzyme and Novartis. M.P.W. has received consultancy fees from Biogen, Roche and Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The MS Centre Amsterdam is supported by the Dutch MS Research Foundation (grant number 14-358e). Frederik Barkhof (FB) is supported by the NIHR UCLH biomedical research centre.
Contributor Information
Iris Dekker, Department of Radiology and Nuclear Medicine, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands/Department of Neurology, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
Madeleine H Sombekke, Department of Neurology, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
Birgit I Witte, Department of Epidemiology and Biostatistics, VU University Medical Centre, Amsterdam, The Netherlands.
Jeroen JG Geurts, Department of Anatomy & Neuroscience, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
Frederik Barkhof, Department of Radiology and Nuclear Medicine, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands/Institutes of Neurology and Healthcare Engineering, University College London, London, UK.
Bernard MJ Uitdehaag, Department of Neurology, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
Joep Killestein, Department of Neurology, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
Mike P Wattjes, Department of Radiology and Nuclear Medicine, Neuroscience Amsterdam, MS Centre Amsterdam, VU University Medical Centre, Amsterdam, The Netherlands.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372(9648): 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. Thouvenot E. Update on clinically isolated syndrome. Presse Med 2015; 44(4 Pt 2): e121–e136. [DOI] [PubMed] [Google Scholar]
- 3. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wattjes MP, Rovira A, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis–establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015; 11(10): 597–606. [DOI] [PubMed] [Google Scholar]
- 5. Rovira A, Wattjes MP, Tintore M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol 2015; 11(8): 471–482. [DOI] [PubMed] [Google Scholar]
- 6. Dekker I, Wattjes MP. Brain and spinal cord MR imaging features in multiple sclerosis and variants. Neuroimaging Clin N Am 2017; 27(2): 205–227. [DOI] [PubMed] [Google Scholar]
- 7. Sombekke MH, Wattjes MP, Balk LJ, et al. Spinal cord lesions in patients with clinically isolated syndrome: A powerful tool in diagnosis and prognosis. Neurology 2013; 80(1): 69–75. [DOI] [PubMed] [Google Scholar]
- 8. Korteweg T, Tintore M, Uitdehaag B, et al. MRI criteria for dissemination in space in patients with clinically isolated syndromes: A multicentre follow-up study. Lancet Neurol 2006; 5(3): 221–227. [DOI] [PubMed] [Google Scholar]
- 9. Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76(8): 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swanton JK, Fernando KT, Dalton CM, et al. Early MRI in optic neuritis: The risk for disability. Neurology 2009; 72(6): 542–550. [DOI] [PubMed] [Google Scholar]
- 11. Zecca C, Disanto G, Sormani MP, et al. Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler 2016; 22(6): 782–791. [DOI] [PubMed] [Google Scholar]
- 12. Brex PA, O’Riordan JI, Miszkiel KA, et al. Multisequence MRI in clinically isolated syndromes and the early development of MS. Neurology 1999; 53(6): 1184–1190. [DOI] [PubMed] [Google Scholar]
- 13. O’Riordan JI, Losseff NA, Phatouros C, et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndromes suggestive of demyelination. J Neurol Neurosurg Psychiatry 1998; 64(3): 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arrambide G, Rovira A, Sastre-Garriga J, et al. Spinal cord lesions: A modest contributor to diagnosis in clinically isolated syndromes but a relevant prognostic factor. Mult Scler 2018; 24: 301–3112. [DOI] [PubMed] [Google Scholar]
- 15. Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014; 9(3): e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brownlee WJ, Altmann DR, Da Mota PA, et al. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult Scler 2017; 23: 665–674. [DOI] [PubMed] [Google Scholar]
- 17. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol 1983; 13(3): 227–231. [DOI] [PubMed] [Google Scholar]
- 18. Lukas C, Sombekke MH, Bellenberg B, et al. Relevance of spinal cord abnormalities to clinical disability in multiple sclerosis: MR imaging findings in a large cohort of patients. Radiology 2013; 269(2): 542–552. [DOI] [PubMed] [Google Scholar]
- 19. Daams M, Weiler F, Steenwijk MD, et al. Mean upper cervical cord area (MUCCA) measurement in long-standing multiple sclerosis: Relation to brain findings and clinical disability. Mult Scler 2014; 20(14): 1860–1865. [DOI] [PubMed] [Google Scholar]
- 20. Coret F, Bosca I, Landete L, et al. Early diffuse demyelinating lesion in the cervical spinal cord predicts a worse prognosis in relapsing-remitting multiple sclerosis. Mult Scler 2010; 16(8): 935–941. [DOI] [PubMed] [Google Scholar]