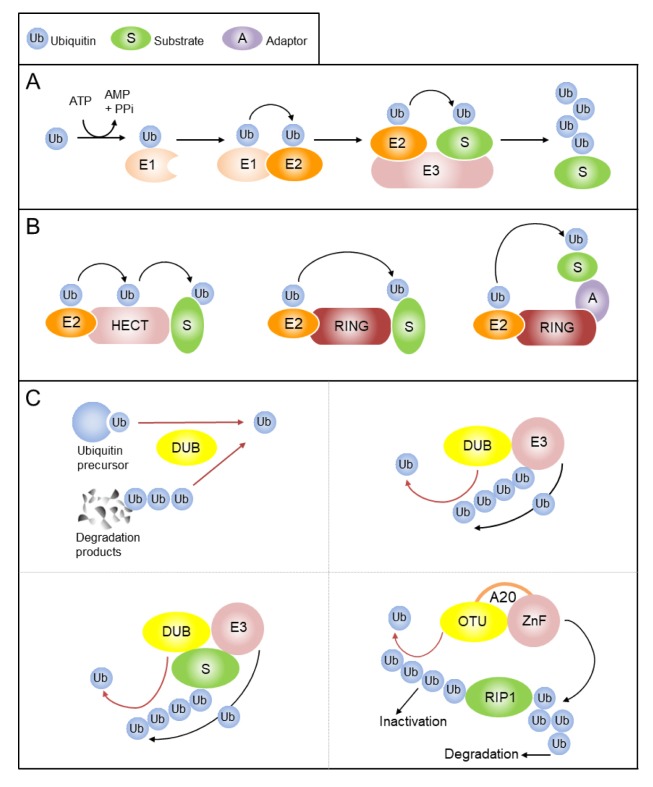
Fig. 1.
The ubiquitin system. (A) Ubiquitin is activated by a ubiquitin-activating enzyme (E1) and transferred to a ubiquitin-conjugating enzyme (E2). E3 ligase binds target substrate and coordinates the covalent attachment of ubiquitin. Target proteins may be mono-ubiquitinated or, as in this example, poly-ubiquitinated. (B) The HECT E3 ligase acts as an acceptor of ubiquitin from E2 enzyme. Ubiquitin is then transferred to a specific Lys residue in the substrate. Conversely, RING E3 ligase acts as scaffold by facilitating interaction between E2 and substrate. These E3s can be a single chain or as large multiprotein complexes. (C) Generation of free ubiquitin from the precursors is a key function of deubiquitinases (DUBs). DUBs have a crucial role in maintaining ubiquitin homeostasis and preventing degradation of ubiquitin together with substrates of the proteasomal or lysosomal pathways (recycling of ubiquitin) (upper left panel). DUB-E3 interactions can rescue E3s or common substrates from degradation, or remove a non-degradative ubiquitin signal (upper right and lower left panels). The A20 combines DUB activity with E3 activity in one single polypeptide chain to modulate the ubiquitination status of key adaptors in NF-κB signaling (lower right panel). E3 ligase and DUB activity is indicated with black arrows and red arrows, respectively.