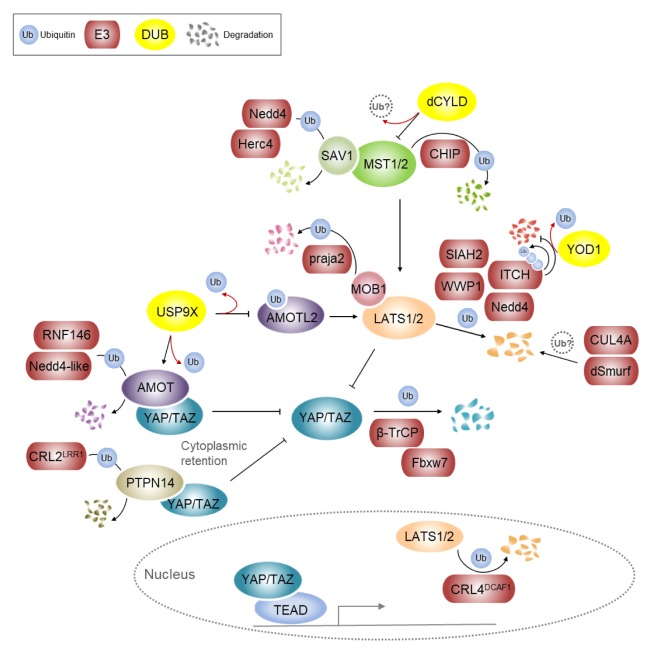
Fig. 2.
Ubiquitin-mediated regulation of the core Hippo pathway components. E3 ligase CHIP targets MST1/2 for ubiquitination. Drosophila CYLD (dCYLD) decreases Hpo (Drosophila ortholog of MST1/2) activity, but it is necessary to investigate whether the suppression is due to deubiquitinase activity of dCYLD. Nedd4 and Herc4 induce SAV1 ubiquitination and degradation in mammals and Drosophila, respectively. Multiple E3 ligases, such as Nedd4, ITCH, SIAH2 and WWP1, target LATS1/2 for degradation, thus negatively regulate Hippo signaling. The activity of LATS1 or level of LATS2 is also regulated in the nucleus by CRL4DCAF1. The activity of LATS1/2 can be indirectly modulated by E3 ligase or DUB. The deubiquitination of AMOTL2 by USP9X depresses LATS1/2 activity and subsequently activates YAP/TAZ. Praja2-mediated ubiquitination targets MOB1 for degradation which leads to inactivation of LATS1/2. β-TrCP and Fbxw7 mediate the ubiquitination of YAP/TAZ, and thus their subsequent proteasomal degradation. Both AMOT and PTPN14 as interacting proteins of YAP/TAZ can undergo ubiquitin-mediated proteolysis by each different E3 ligases, and thus allow YAP to translocate into nuclei. In contrast, deubiquitination of AMOT by USP9X results in stabilization of AMOT and retention of YAP in the cytoplasm, which leads to lower YAP/TAZ activity. E3 ligase and DUB activity is indicated with black arrows and red arrows, respectively.