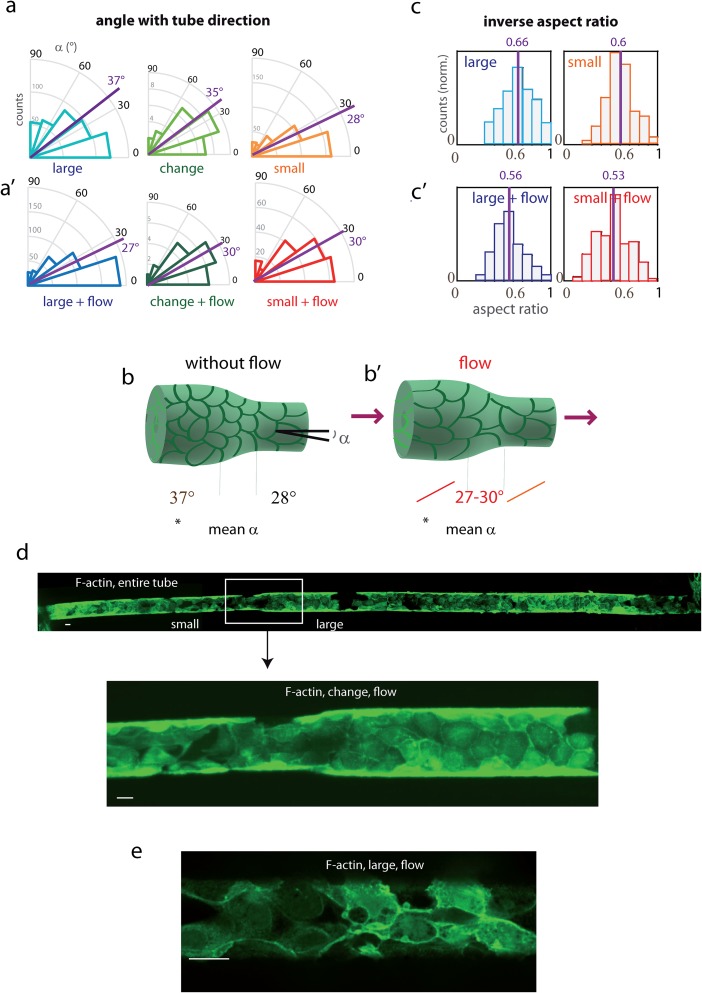
FIG. 3.
Orientation and elongation of cells in tubes with or without flow. (a) Cell orientation and elongation in the different tube parts: (a) angles with the tube axis of the cell principal direction, measured in the different parts of tubes: large-diameter (blue), change in diameter (green), and small-diameter (orange). All measurements were made in fibronectin-coated tubes, 3–5 days after cell seeding. Large and small-diameters data were statistically significantly different with p < 10−4 (see Table I). Mean angles are indicated in magenta. (a′) Similar diagrams after one day of continuous flow (0.15 μl/min). (b) and (b′) Recapitulative scheme of orientation as a function of tube diameter, in static conditions (left) and under flow (right). (c) and (c′) Histograms of the inverse aspect ratio (length of short axis divided by the length of the long axis) (c) in static conditions: large-diameter section (blue), small-diameter section (orange), and (c′) after 1 day of continuous flow: large-diameter section (dark blue), small-diameter section (red). Counts (y axis) were normalized so that the histogram area was the same for the different conditions. See Table I for statistics. (a to c′) Organization in the large-diameter section in static conditions systematically differed from other conditions, with statistically significant differences, p < 10−4. Mean ratios are indicated in magenta. (d) Whole-tube image of MDCK Lifeact-GFP cells in the fibronectin-coated tube upon flow (0.15 μl/min). Imaging performed at the spinning disk with a ×10 objective (top, concatenated fields, bottom, detail). After seeding, the cells were kept 2 days in static conditions in order to achieve optimal binding, and a 1-day flow was applied. (b) zoom on the region of change in diameter. The bar represents 20 μm. (e) F-actin organization (Lifeact-GFP labeling, lower part of the tube) after 1-day flow, imaged with a 60× objective, spinning disk.